Recent Advances in Polyphenol Research
Здесь есть возможность читать онлайн «Recent Advances in Polyphenol Research» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Recent Advances in Polyphenol Research
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Recent Advances in Polyphenol Research: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Recent Advances in Polyphenol Research»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Recent Advances in Polyphenol Research
Recent Advances in Polyphenol Research — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Recent Advances in Polyphenol Research», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
3 Dual connectivity (A‐type): Figure 2.3shows a rarer class of oligomeric PAs, in which all or part of the monomer units are doubly connected through a C–C bond and a C–O bond, thereby forming a characteristic dioxabicyclo[3.3.1]nonane skeleton. Such a dual interflavan connectivity is called an A‐type. These compounds constitute a small but potentially interesting molecular class. As represented by section III of Figure 2.3, most congeners of this class have a C–C bond [C(4)–C(8)] and a C–O bond {C(2)–O[C(7)]}. An increasing number of the natural PAs with the A‐type linkages has been identified. Taking the trimers as examples in Figure 2.3, cinnamtannin B1 has one double linkage and one single linkage, while aesculitannin C has two double linkages. For some other higher‐order structures, see Figure 2.6(vide infra). In addition, there are minor congeners having a different dual connectivity; i.e. a C–C bond [C(4)–C(6)] and a C–O bond {C(2)–O[C(7)]} as represented by section IV of Figure 2.3.

Figure 2.2 Origin of the structural diversity in oligomeric PAs: B‐type connectivity.
This chapter will focus on the oligomeric PAs with an A‐type structure. Section 2.2explores the history of the structure elucidation, and section 2.3summarizes the postulated biosyntheses and the model studies for construction of the dioxabicyclo[3.3.1]nonane skeleton, with several examples of the completed total syntheses.
2.2 Structure
This section describes a brief history of the oligomeric PAs with A‐type linkages. In 1966, Mayer and coworkers isolated a crystalline phenolic compound from the seed shells of a horse chestnut ( Aesculus hippocastanum L.). Using mass spectroscopy, the chemical composition was determined to be C 30H 24O 12(Mayer et al. 1966). The compound was hydrolyzed with conc. hydrochloric acid, and paper chromatography identified two hydrolysates, epicatechin ( 1) and cyanidin ( 2) ( Figure 2.4). At this stage, the compound was named procyanidino‐(–)‐epicatechin without specifying the parent dimer structure. Three possible dimeric structures, I– III, were postulated, sharing a common feature that two epicatechin units are linked through a double linkage. They stated that, among these, the structure IIIwould be most likely.
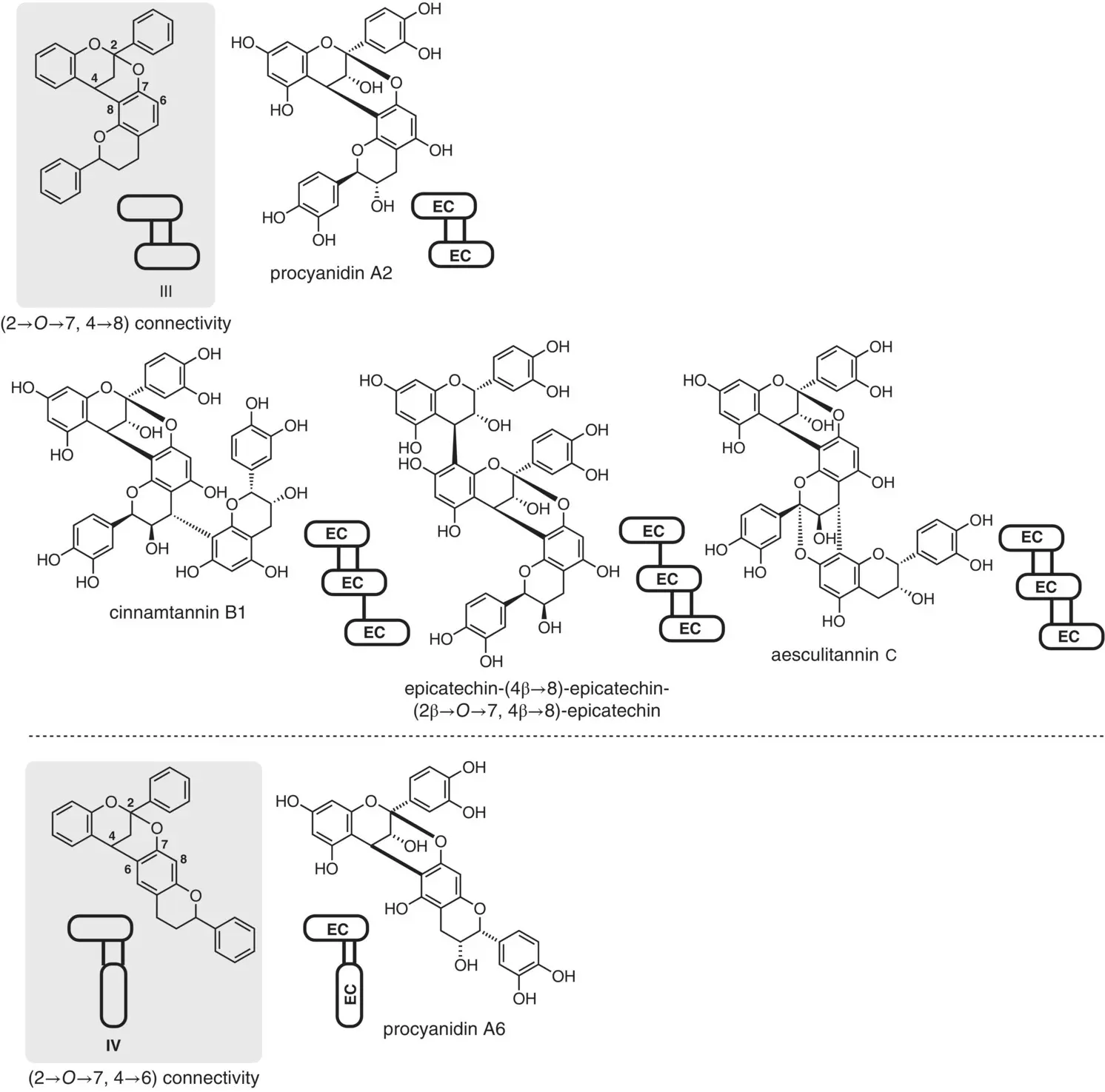
Figure 2.3 Origin of the structural diversity in oligomeric PAs: compounds having the A‐type connectivity.
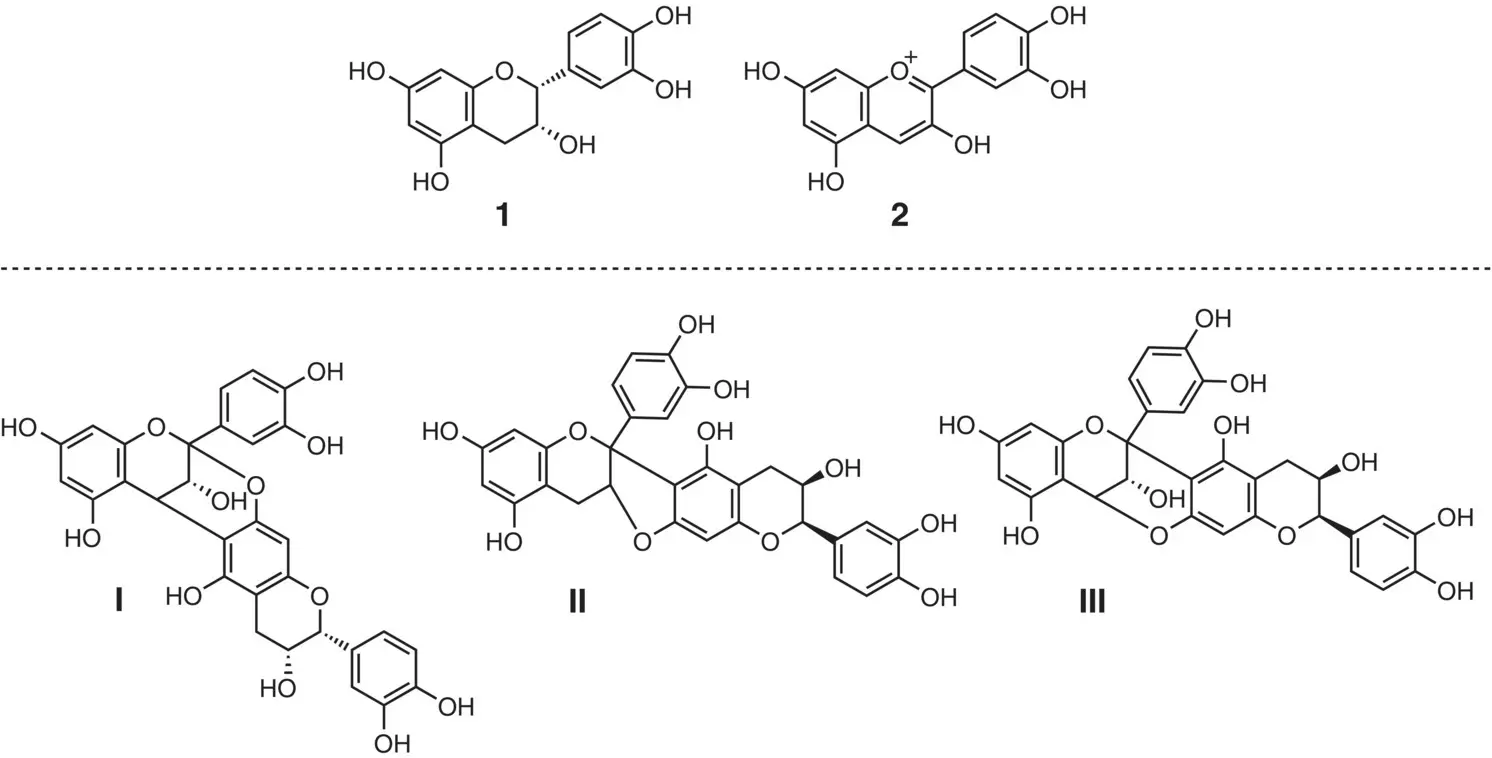
Figure 2.4 Acid hydrolysates of the dimeric proanthocyanidin isolated from Aesculus hippocastanum , and three possible dimeric structures proposed by Mayer.
Later, Weinges and coworkers extracted several related compounds from cranberries ( Vaccinium vitis‐idaea ), which included the “Mayer's dimer” mentioned above (Weinges et al. 1968). They arbitrarily classified those compounds into two categories by the number of hydrogen atoms ( Figure 2.5): the dimeric proanthocyanidin compounds with molecular formula C 30H 24O 12as the A‐type, and those with C 30H 26O 12as the B‐type. Thus, the above‐stated Mayer's compound, i.e. procyanidino‐(–)‐epicatechin, was renamed procyanidin A2.
Haslam and coworkers later proposed the currently accepted structure 3( Figure 2.5a) with a characteristic bicyclic skeleton formed by two interflavan bonds {C(4)–C(8) and C(2)–O[C(7)]} based on the 1H‐ and 13C‐NMR data and some chemical evidences (Jacques et al. 1973, 1974). A decade later, the structure was unequivocally verified by X‐ray crystallography (Van Rooyen and Redelinghuys 1983).
With advances in the separation and analytical methods, the more complicated oligomeric PAs having A‐type linkages have been identified from various plant sources. Figure 2.6shows some of the tetramers with A‐type structure, which are homo‐ or hetero‐oligomers (Nonaka et al. 1983; Morimoto et al. 1985; Morimoto et al. 1987; Balde et al. 1995; Nam et al. 2017). It should be noted that the molecular diversity arises not only from the elements stated before, but also from the inclusion of the enantiomeric flavan‐3‐ols (e.g. ent ‐AZ, ent ‐EC, ent ‐CA in pavetannin C5) as constituent monomers. The diversity will increase in the future, and some of these compounds may show potentially significant biological activities.
2.3 Synthetic Studies
2.3.1 Hypothetical Biosynthetic Routes
On the biogenesis of the characteristic double linkages (A‐type), two putative pathways have been proposed (Paths I and II, Figure 2.7) (Selenski and Pettus 2006). Different consequences would be expected on the reactivity and the stereochemistry in the formation of the [3.3.1]bicyclo skeleton D. Path I entails the addition of the flavan nucleophile Bto the electrophilic partner A, producing the singly linked dimer C(the B‐type structure). Oxidation at the C(2) position of the upper flavan unit in Callows the formation of a doubly linked derivative D(the A‐type structure). From the stereochemical standpoint, if the initial C–C bond is formed in a stereoselective manner, the stereochemistry generated by the subsequent C–O bond formation would be settled spontaneously, due to the steric constraints in the bicyclic skeleton. On the other hand, Path II is based on a formal [3+3]‐cycloaddition of the flavylium Ewith the flavan unit B. Since the flavylium Elacking any stereogenic centers is achiral (prochiral), the [3+3]‐cycloaddition reaction needs to proceed with enantiofacial selectivity, which may be regulated by enzymes in the biogenesis. These putative biosynthetic pathways would give hints to chemical synthesis.
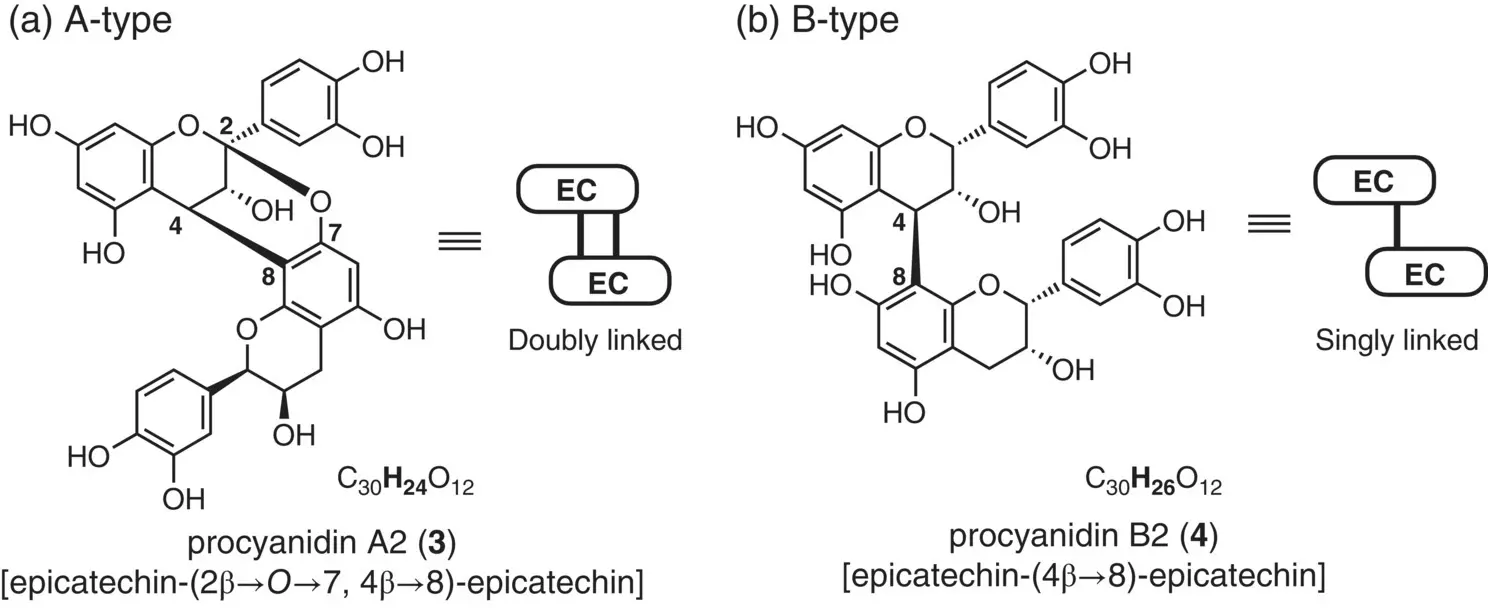
Figure 2.5 Mayer's PA (procyanidin A2): the terminological origin of A/B‐type structures.

Figure 2.6 Structures of the tetramers with A‐type linkages.
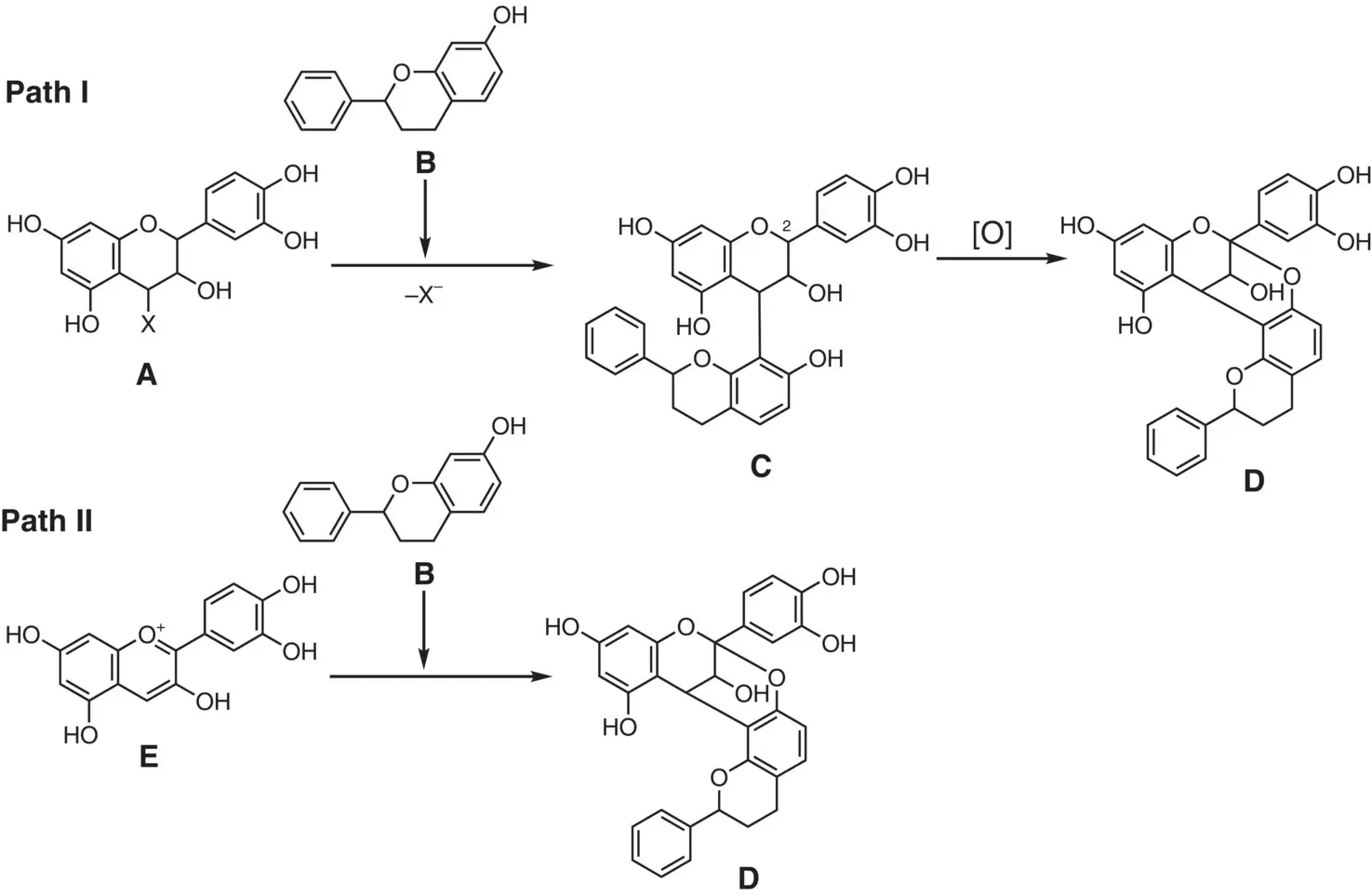
Figure 2.7 Two plausible biosynthetic pathways forming the A‐type structure.
2.3.2 Retrosynthesis
Figure 2.8illustrates a synthetic analysis en route to the A‐type structure, focusing on the key dioxabicyclic skeleton. Three potential pathways are shown.
Route I is relevant to the Path I biosynthesis discussed in Section 2.3.1(see Figure 2.7), disconnecting the C–O bond i in Ato Bwith a single connection and the C(2) cation center, which could be traced back to a B‐type structure B'as a precursor. In executing the synthesis, this approach has an advantage, that the corresponding B‐type structures are synthetically well accessible (Ohmori et al. 2004, 2011; Oyama et al. 2008; Kozikowski and Tückmantel 2009; Saito et al. 2009; Yano et al. 2012; Makabe 2013). However, a concern is that the site‐specific oxidation at the C(2) benzylic center on the upper flavan unit may be challenging.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Recent Advances in Polyphenol Research»
Представляем Вашему вниманию похожие книги на «Recent Advances in Polyphenol Research» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Recent Advances in Polyphenol Research» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












