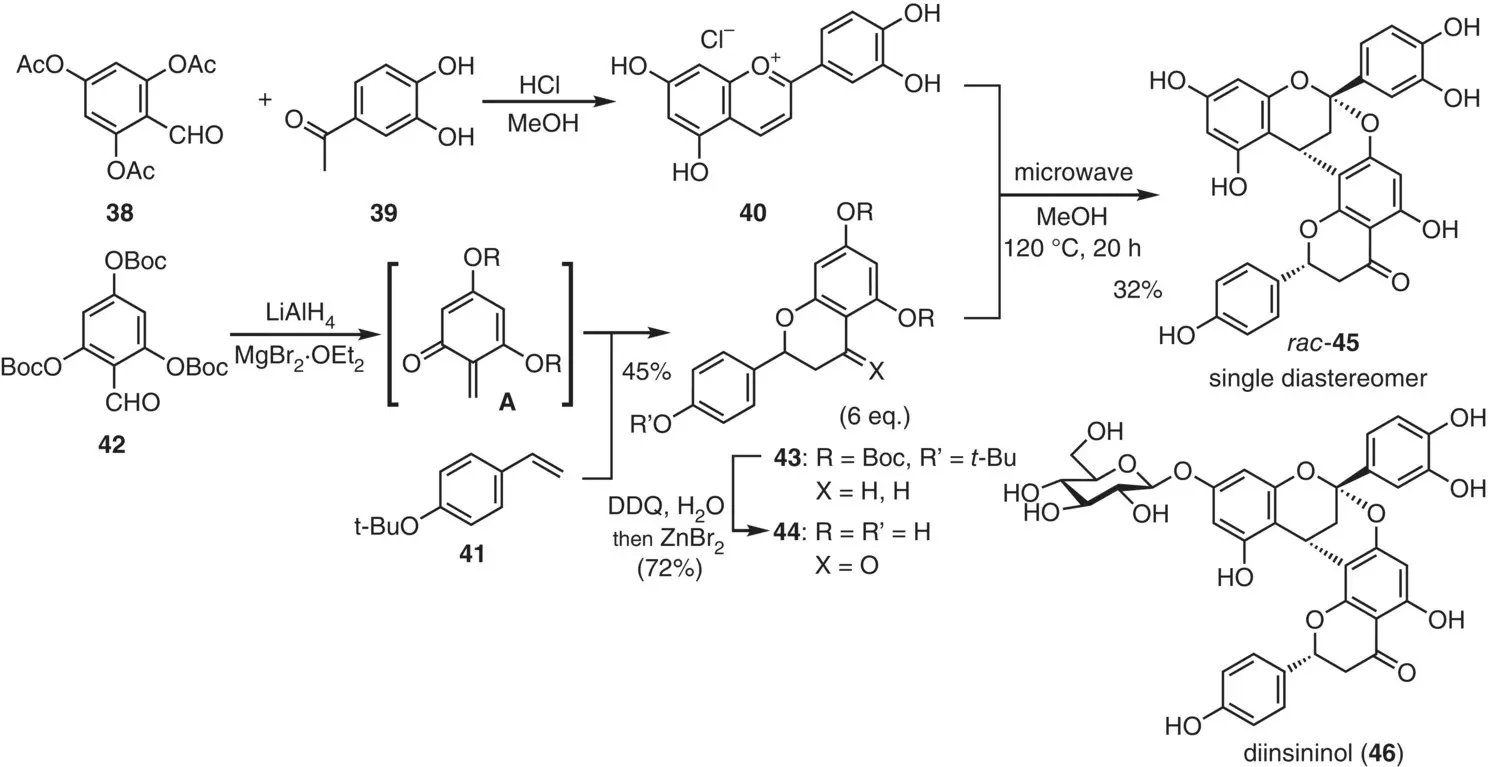
Figure 2.21 Pettus's diinsininol aglycon synthesis.
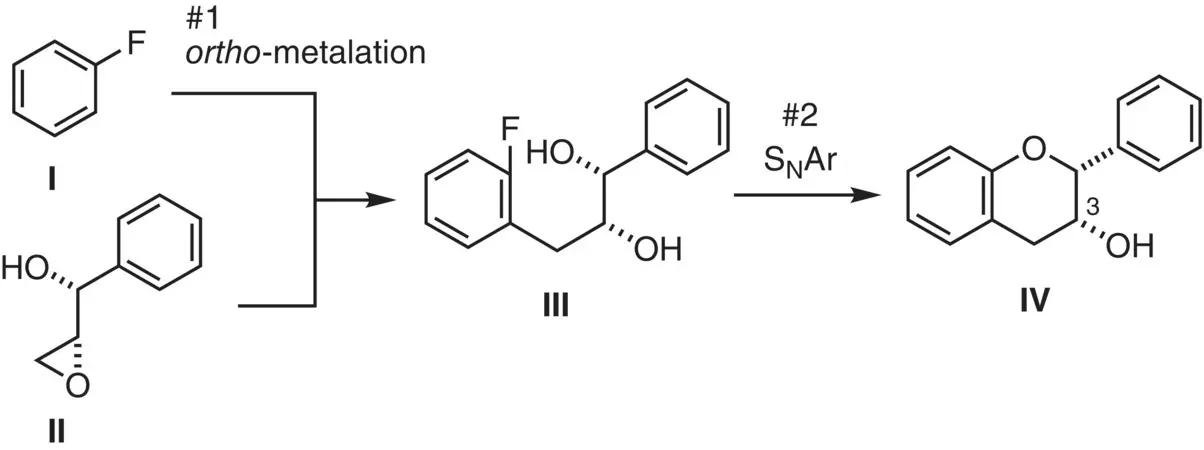
Figure 2.22 Strategy for monomer synthesis.
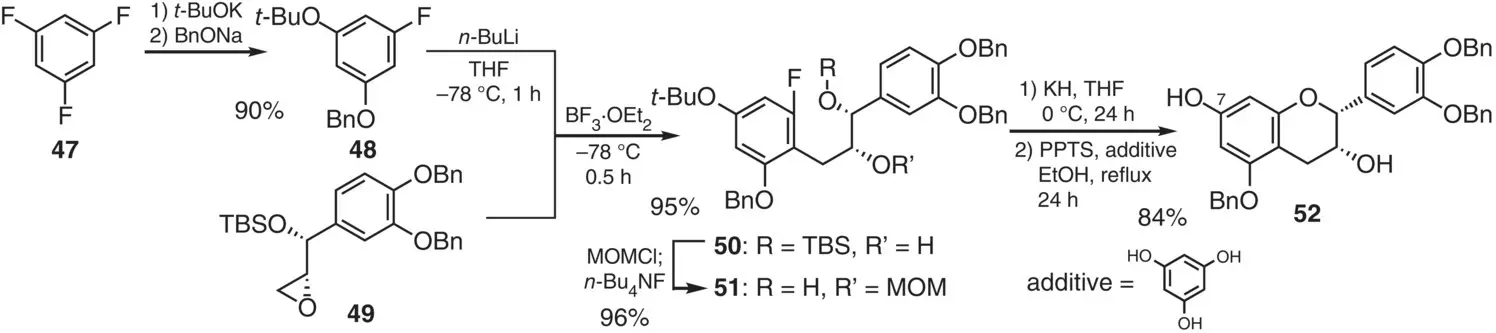
Figure 2.23 De novo synthesis of the C(7)‐hydroxy monomer unit.
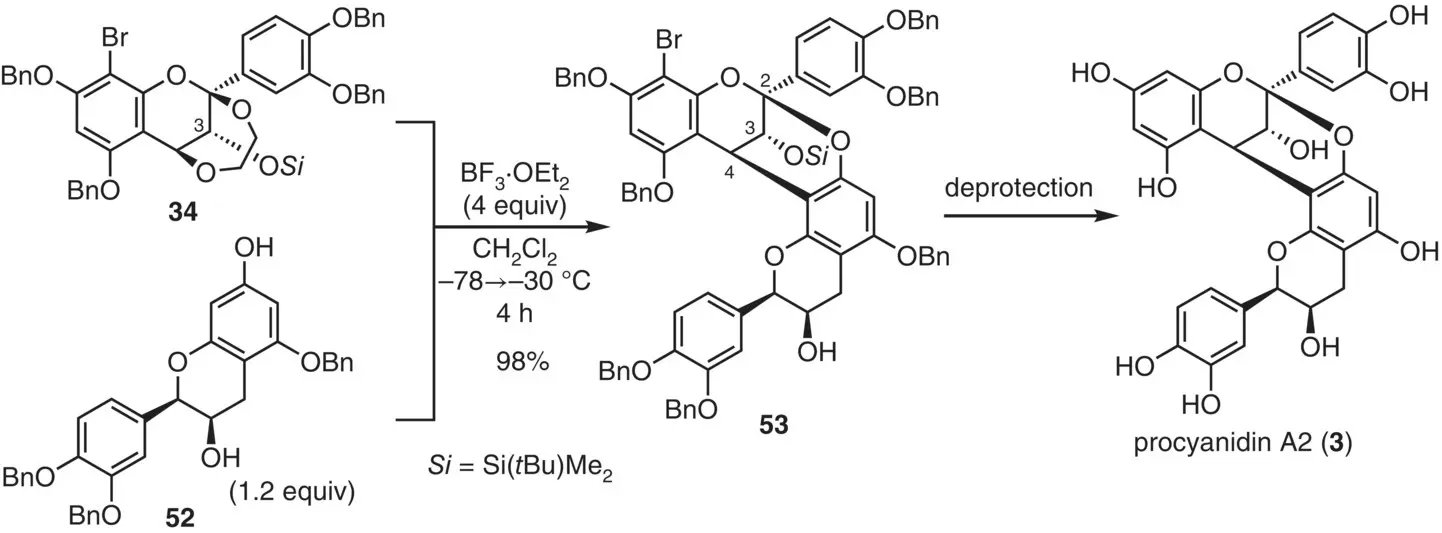
Figure 2.24 Total synthesis of procyanidin A2 via flavan annulation.
More recently, it was found that the annulation could work even with a non‐protected flavan unit, e.g. catechin or epicatechin, as a nucleophile ( Figure 2.25) (Betkekar et al. 2019). In a solvent mixture of ethanol and 1,4‐dioxane, (–)‐epicatechin was allowed to react with electrophilic flavan unit 54in the presence of camphor sulfonic acid, affording annulation product 55along with minute amounts of its regioisomers 56and 57. After chromatographic separation (silica gel) and deprotection, all products were converted to their free‐forms, i.e. procyanidin A2 ( 3), proanthocyanidin A6, and proanthocyanidin A7.

Figure 2.25 Annulation with a free flavan unit and syntheses of procyanidin A2, and proanthocyanidin A6 and A7.
These results indicate that the free flavan unit preferentially reacted at its C(8) position rather than the C(6) position. To address the origin of this C8/C6‐regioselectivity, a DFT calculation was conducted on the putative Wheland intermediates IIand III, generated by the reaction on the model substrate Iwith CH 3 +( Figure 2.26). The result suggested that the C8‐methyl intermediate IIis energetically more stable over the C6‐counterpart III.
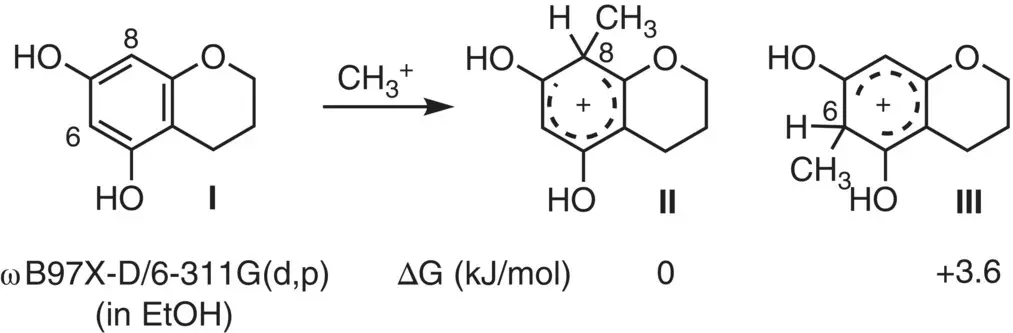
Figure 2.26 DFT calculations of the Wheland intermediates, IIand III, derived from model compound I.
2.3.6.3 Synthesis of (+)‐Cinnamtannin B1 (62)
This annulation method was also applied to the synthesis of a trimeric natural product, (+)‐cinnamtannin B1 ( 62) ( Figure 2.27) (Ito et al. 2014), which is known as a rare sweet polyphenol. To realize the convergent assembly of the trimeric scaffold, the orthogonal coupling strategy was adapted by employing monomers 34, 58, and 52. Annulation of dioxy electrophilic unit 34with sulfide 58was carried out by using BF 3·OEt 2to give the coupling product 59in 91% yield. The arylthio group remained intact under the hard Lewis acidic conditions. Next, the soft activation of dimeric sulfide 59was conducted by using I 2and Ag 2O, enabling the reaction with the nucleophilic epicatechin unit 52to give trimer 60in 96% yield. After two‐step deprotection via 61, (+)‐cinnamtannin B1 ( 62) was obtained in 70% overall yield.
2.3.6.4 Synthesis of Selligueain A (63)
The coupling protocols were applied to the synthesis of selligueain A ( 63), isolated from the rhizomes of Selliguea feei ( Figure 2.28) (Noguchi et al. 2018). Although this compound is interesting because of the pronounced sweetness, 35 times sweeter than sucrose, it had been obtained in only minute amounts from nature. A serious issue was that the trimers are comprised of two rare flavan monomer units, i.e. (–)‐epiafzelechin (EZ) and (+)‐afzelechin (AZ), which manifests a general bottleneck in the chemical synthesis of flavan oligomers – that is, the limited availability of their monomeric constituents. Note that commercially available flavan‐3‐ol is basically limited to (+)‐catechin, and other congeners are scarce and could not be used as the starting materials.
The initial step in the assembly of these monomer units was the annulation of the top unit 64and the middle unit 65by using BF 3·OEt 2to give 66as a sole product in 86% yield ( Figure 2.29). Importantly, no formation of the C(4)→C(6)‐linked products was observed, and the sulfide moiety remained intact under these hard Lewis acidic conditions. After detachment of the silyl group in 66, the arylthio group in the resulting alcohol 67was activated under soft Lewis acidic conditions to react with the bottom unit 68. The annulation reaction proceeded the annulation, giving trimer 69in 93% yield. It should be noted that the seemingly labile benzylic acetal moiety at the C(2) position of the top unit remained untouched. Finally, all benzyl groups and the bromine atom were removed by hydrogenolysis to give pure 63in 85% yield.
2.3.6.5 Synthesis of Procyanidin A2 (3)
As another synthetic approach, Sharma et al. (2015) reported the de novo synthesis of a series of the dimeric PAs having an A‐type structure, which relied on the Friedel–Crafts reaction of two monomeric units followed by internal acetalization ( Figure 2.30). Successive DDQ oxidation of diol 70with/without 2‐ethoxyethanol gave the diastereomeric alkoxy ketones, 72αand 72β, in 28% and 12% yield, respectively.
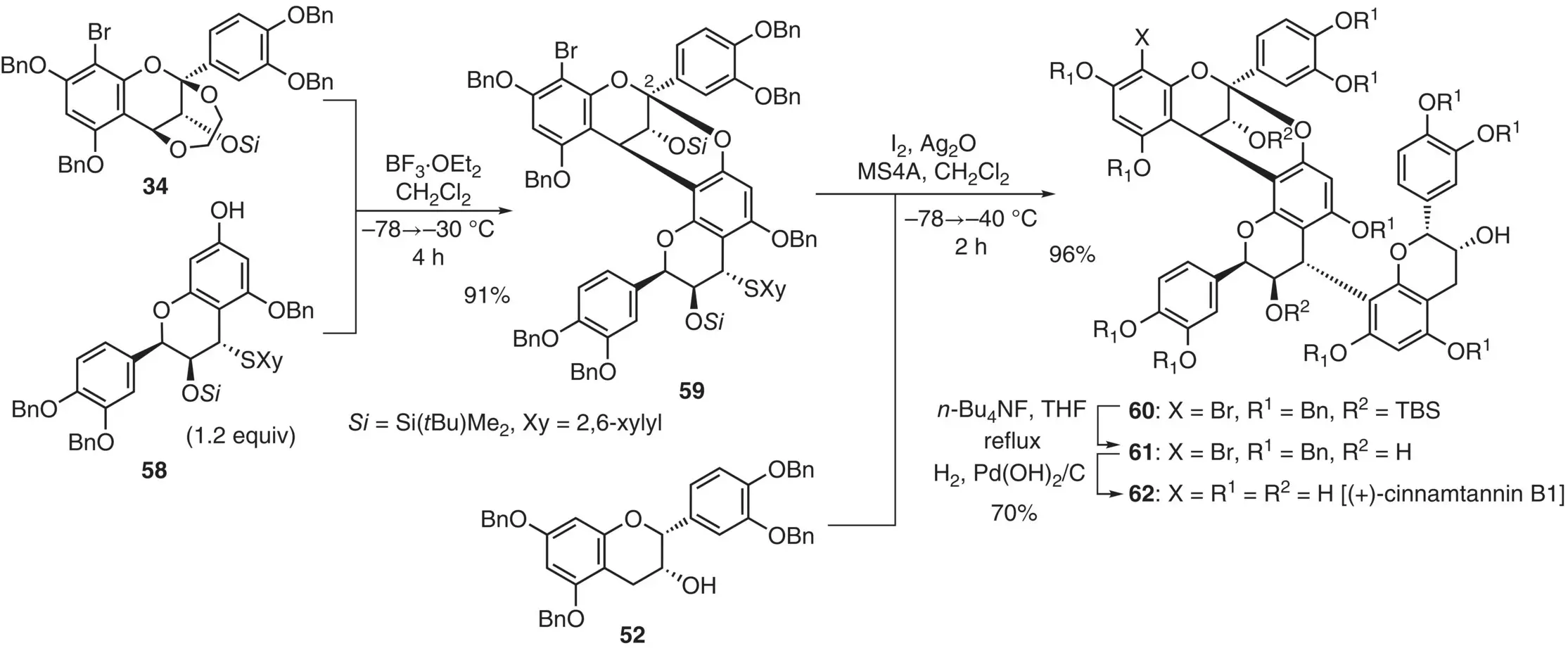
Figure 2.27 Synthesis of cinnamtannin B1 based on the orthogonal activation approach.
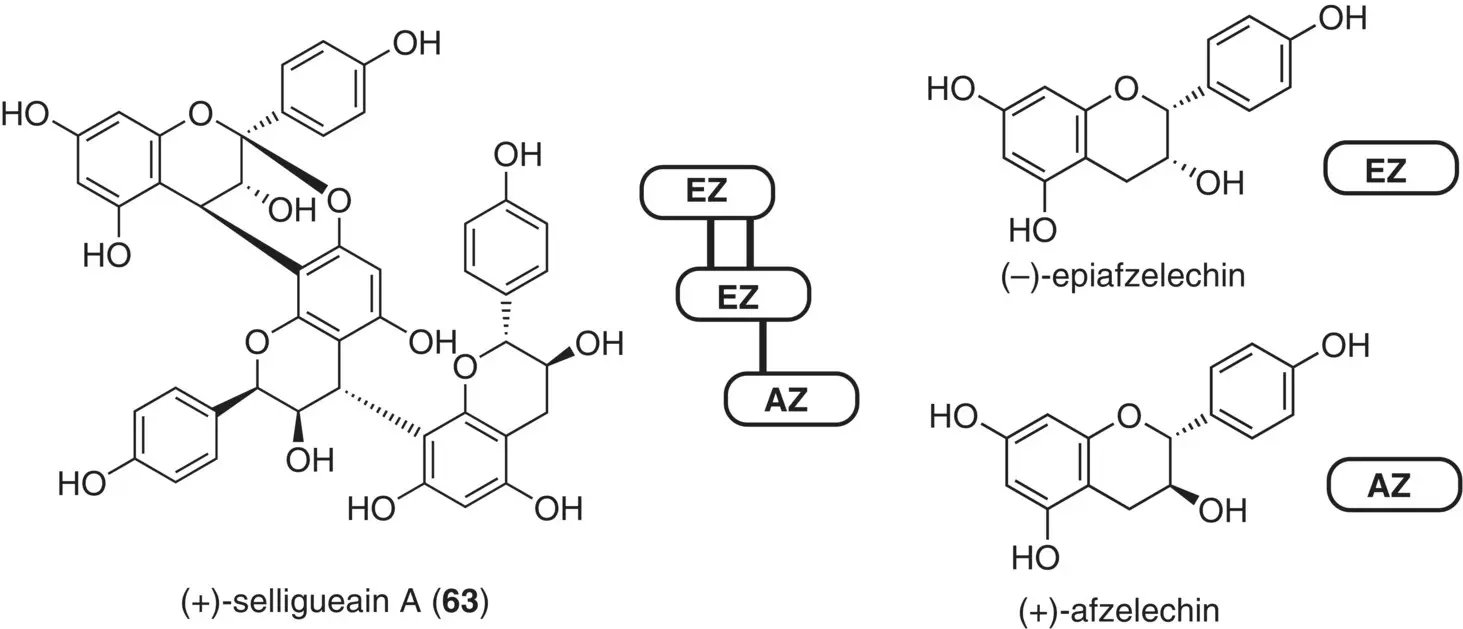
Figure 2.28 Structure of (+)‐selligueain A, its monomeric flavan constituents, (+)‐afzelechin (AZ) and (–)‐epiafzelechin (EZ), and three monomeric synthetic units for orthogonal assembly.
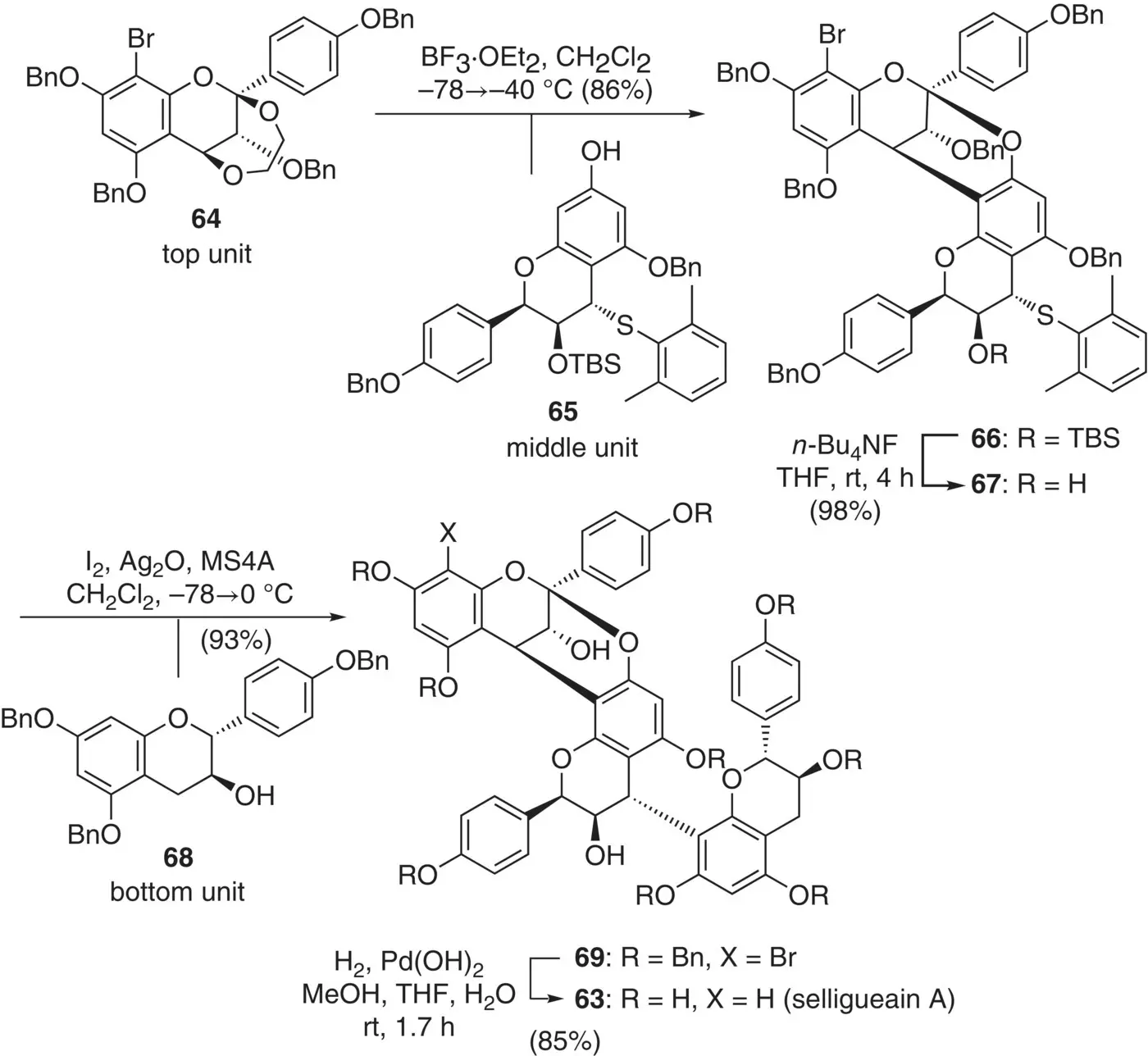
Figure 2.29 Orthogonal activation and synthesis of selligueain A.

Figure 2.30 Synthesis of a series of dimeric PAs having A‐type structure via the Friedel–Crafts reaction.
Читать дальше






















