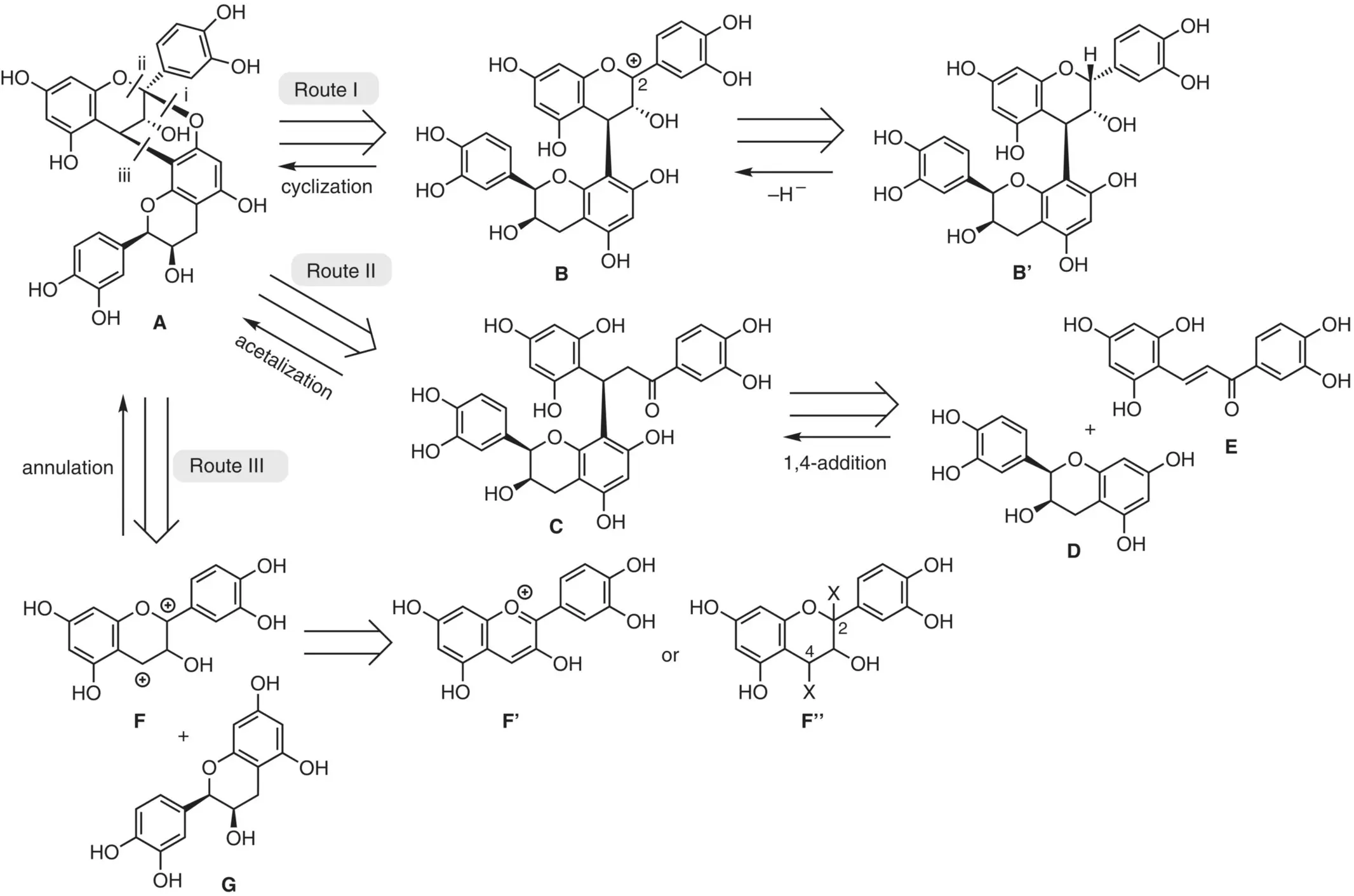
Figure 2.8 Retrosynthetic analyses of the A‐type structure.
Route II relies on the retrosynthetic hydrolysis of the acetal moiety in A. Dissection of the bonds i and ii suggests ketone Cas the precursor. Assuming the Michael addition, ketone Cis accessible by combining an electrophilic chalcone unit Eand a nucleophilic flavan unit D. In this approach, rigorous stereocontrol at the Michael addition stage is necessary.
Route III corresponds to another biomimetic pathway (Path II, Figure 2.7), based on the two‐bond disconnection at bonds i and iii in A, assuming a formal [3+3]‐cycloaddition of a dicationic species Fand a nucleophilic partner G. As the possible synthetic equivalents to the key dicationic species F, one could conceive flavylium salt F'or flavan unit F″with two leaving groups at the C(2) and C(4) positions. This approach would realize direct conversion to the key bicyclic skeleton. If flavylium salt F'were used, the enantiocontrol would inevitably pose a serious problem. In contrast, use of the flavan unit F″could achieve a stereoselective reaction, as will be discussed later (see Section 2.3.5).
2.3.3 Oxidative Conversion from B‐type PAs (Route I)
This section describes the reported reactions based on Route I in Figure 2.8. In early attempts, Nonaka et al. (1987) used the oxidative conversion of the B‐type to the A‐type structure ( Figure 2.9). Upon treatment of procyanidin B1 ( 5) with hydrogen peroxide under basic conditions, an oxidative conversion proceeded to give procyanidin A1 ( 6) in 13% yield. This protocol was further applied to other B‐type structures. For example, procyanidin B5 ( 7) and aesculitannin A ( 10) were converted to the corresponding compounds having the A‐type structure, i.e. procyanidin A7 ( 9) and aesculitannin C ( 11), albeit in low yields.
As other means of converting the B‐type structure into the A‐type structure, a radical‐mediated reaction was exploited ( Figure 2.10) (Kondo et al. 2000). Upon treatment of procyanidin B1 ( 5) with 2,2‐diphenyl‐1‐picrylhydrazyl (DPPH), the C(2) hydrogen atom in the upper epicatechin unit was abstracted, inducing an oxidative cyclization to give procyanidin A1 ( 6), though the chemical yield was not reported.
2.3.4 Approaches via an Acyclic Precursor (Route II)
Weinges and Theobald (1971) reported a stepwise construction of the dioxabicyclo[3.3.1]nonane skeleton (A‐type) of PAs ( Figure 2.11). The Michael addition of the o ‐benzyloxyphenylmagnesium bromide to chalcone 12gave ketone 13. After hydrogenolytic removal of the benzyl protecting groups, the resulting bisphenol was exposed to dehydrating conditions, giving bicycle 14in 50% yield.
Xia et al. (2014) reported a cascade reaction of 2‐hydroxychalcones with phloroglucinol derivatives by using a catalytic amount of ethylenediammonium diacetate (EDDA, 10 mol%), constructing the dioxabicyclo[3.3.1]nonane skeleton ( Figure 2.12). The reaction of 15with 16proceeded in refluxing toluene via the Michael reaction followed by an internal acetal formation, giving the condensation product 17in 87% yield.
Kraus and Geraskin (2017) reported a facile one‐pot formation of the A‐type structure ( Figure 2.13). Under acidic conditions, twofold nucleophilic reactions of phloroglucinol to acetylenic aldehyde 18generated bis‐arylated 19as an intermediate, which underwent acid‐catalyzed acetal formation to give bicycle 20. After acetylation, tetraacetate 21was obtained in high yield.
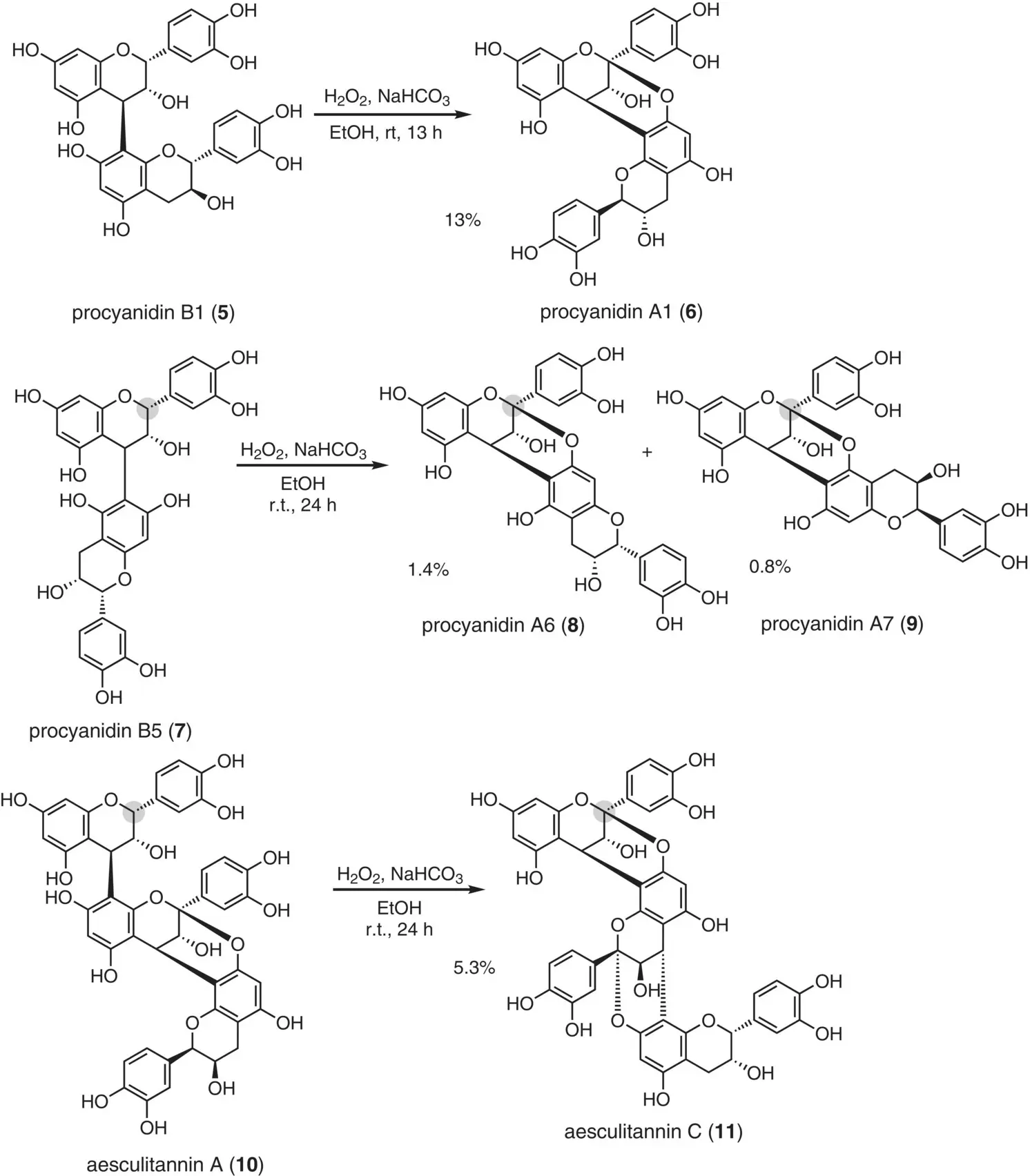
Figure 2.9 Oxidative conversion of the B‐type to the A‐type structure.
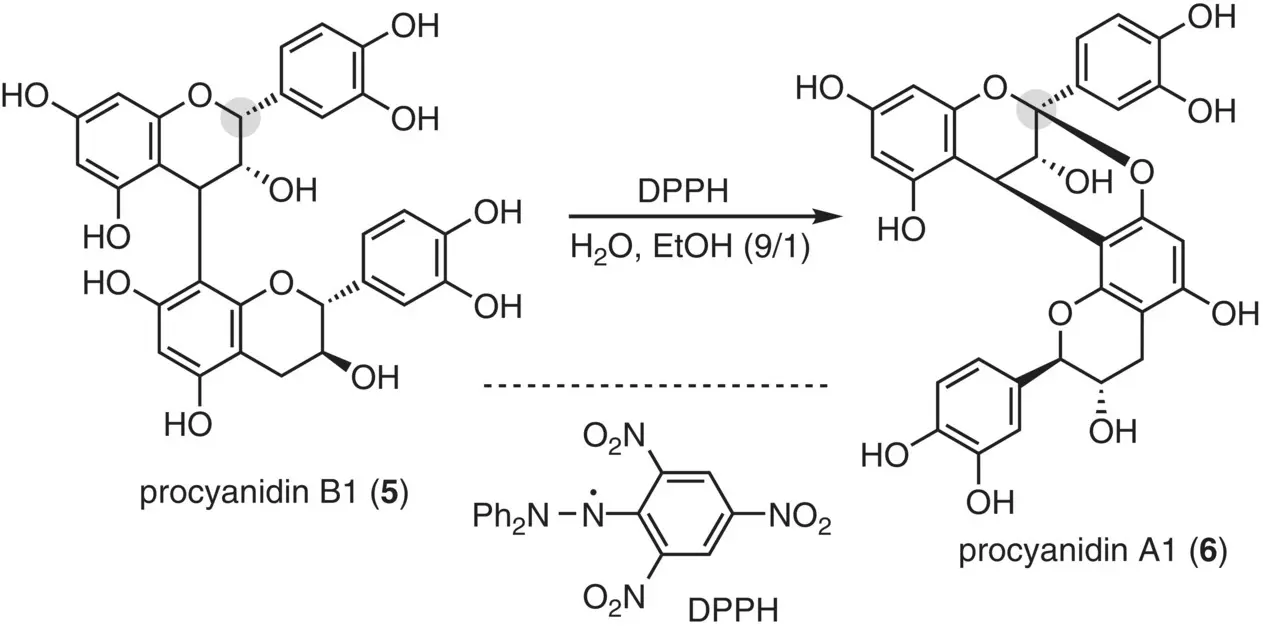
Figure 2.10 Radical‐mediated oxidative conversion.
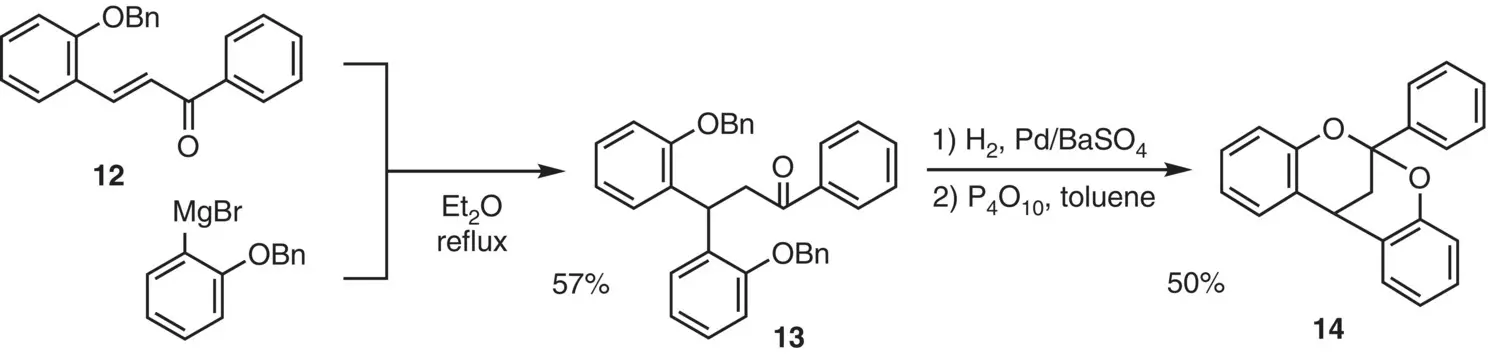
Figure 2.11 Stepwise construction of the A‐type structure.
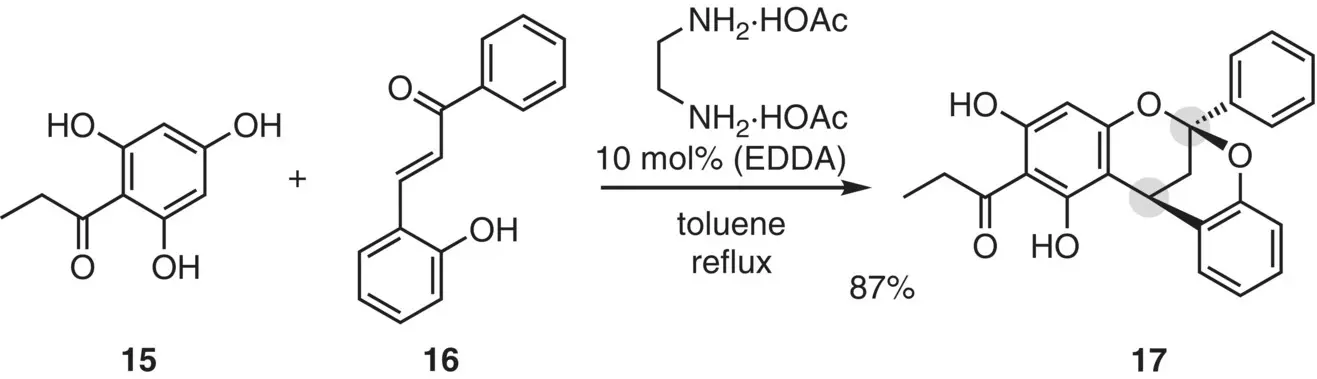
Figure 2.12 Cascade reaction of 2‐hydroxychalcones with a phloroglucinol derivative
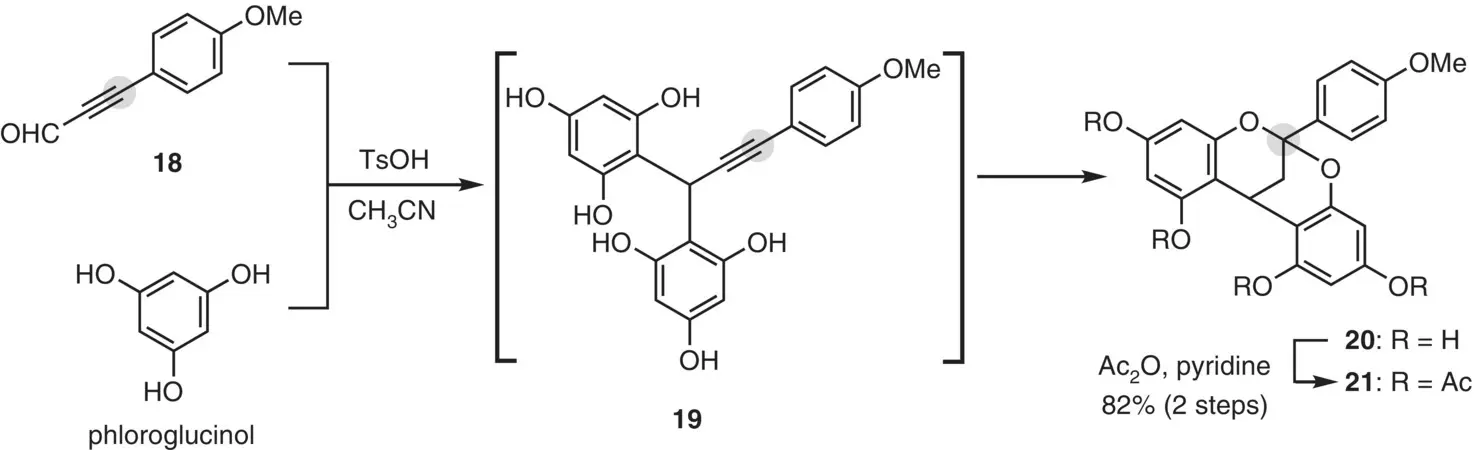
Figure 2.13 One‐pot formation of the A‐type structure.
2.3.5 Annulation Approach (Route III)
For the direct construction of the characteristic dioxabicyclo[3.3.1]nonane skeleton, a pioneering approach was reported by the annulation of flavylium ion 22with phloroglucinol as a nucleophilic unit to form 23( Figure 2.14) (Jurd and Waiss 1965). Treatment of flavylium salt 22with phloroglucinol in aqueous MeOH under weakly acidic conditions (pH 5.8, 60 °C, 15 minutes) gave, after acetylation, the annulation product 23as colorless prisms (23% yield). The stereochemistry was not clarified.
A similar reaction was reported by Pomilio et al. (1977) ( Figure 2.15), carrying out the annulation of flavylium 24and (+)‐catechin under mild acidic conditions (pH 5.8). The reaction was sluggish, and isolation of the product after thorough protection of hydroxy groups resulted in a poor yield of annulation product 25.
Kraus et al. (2009) improved this reaction ( Figure 2.16). Treatment of flavylium salt 24with phloroglucinol followed by treatment with silica gel gave the annulation product 26in good yield. Moreover, use of (+)‐catechin as a nucleophile gave a diastereomer mixture of two annulation products 27aand 27bin 89% combined yield. Recently, another research group reported a similar protocol (Alejo‐Armijo et al. 2018).
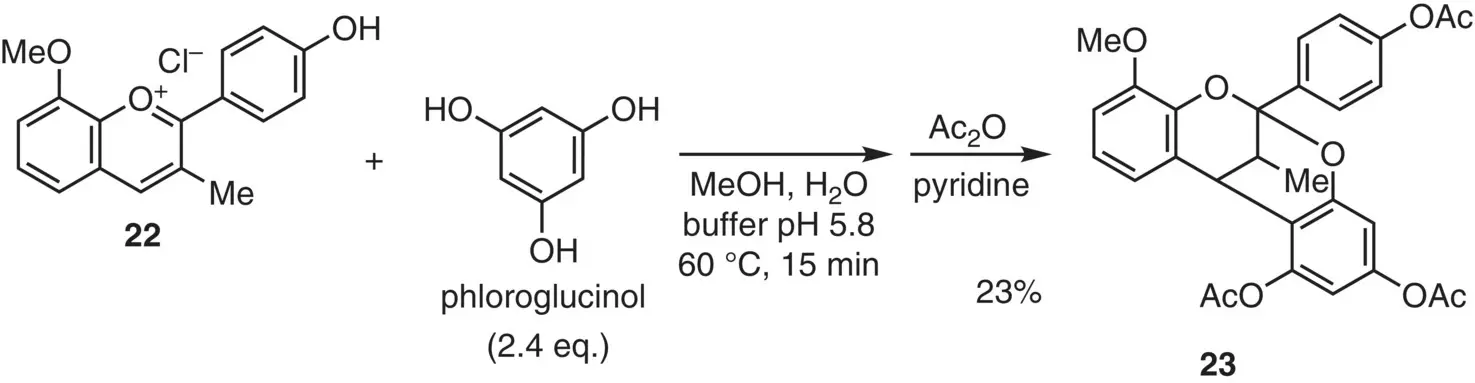
Figure 2.14 Direct annulation approach to the A‐type structure.
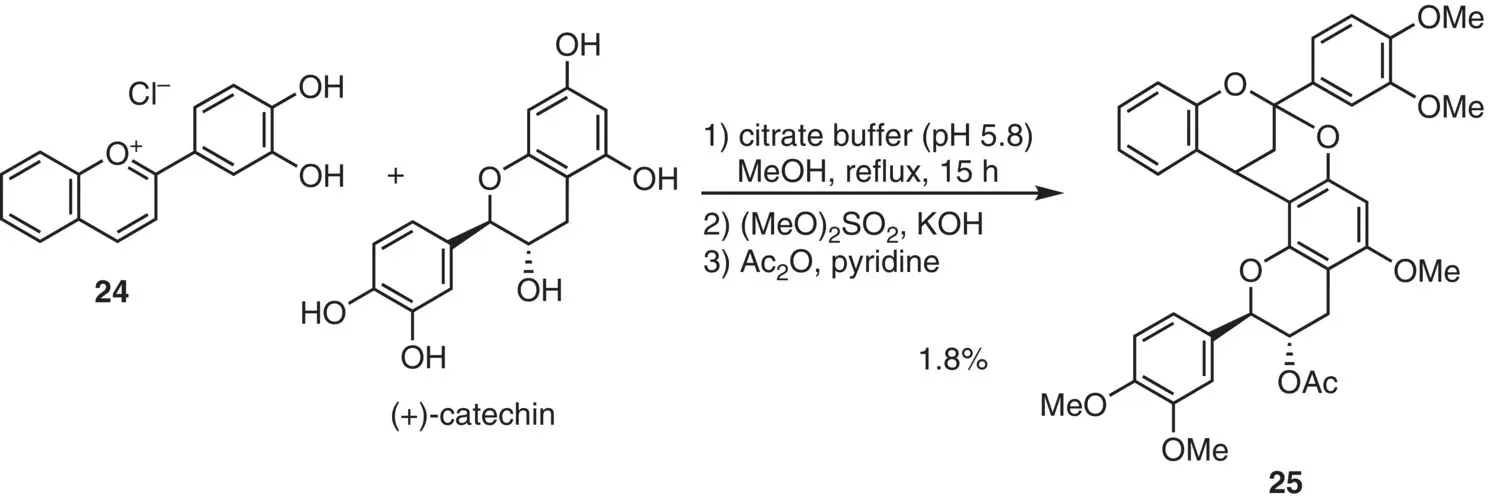
Figure 2.15 Early studies on annulation reaction of flavylium 24with (+)‐catechin.
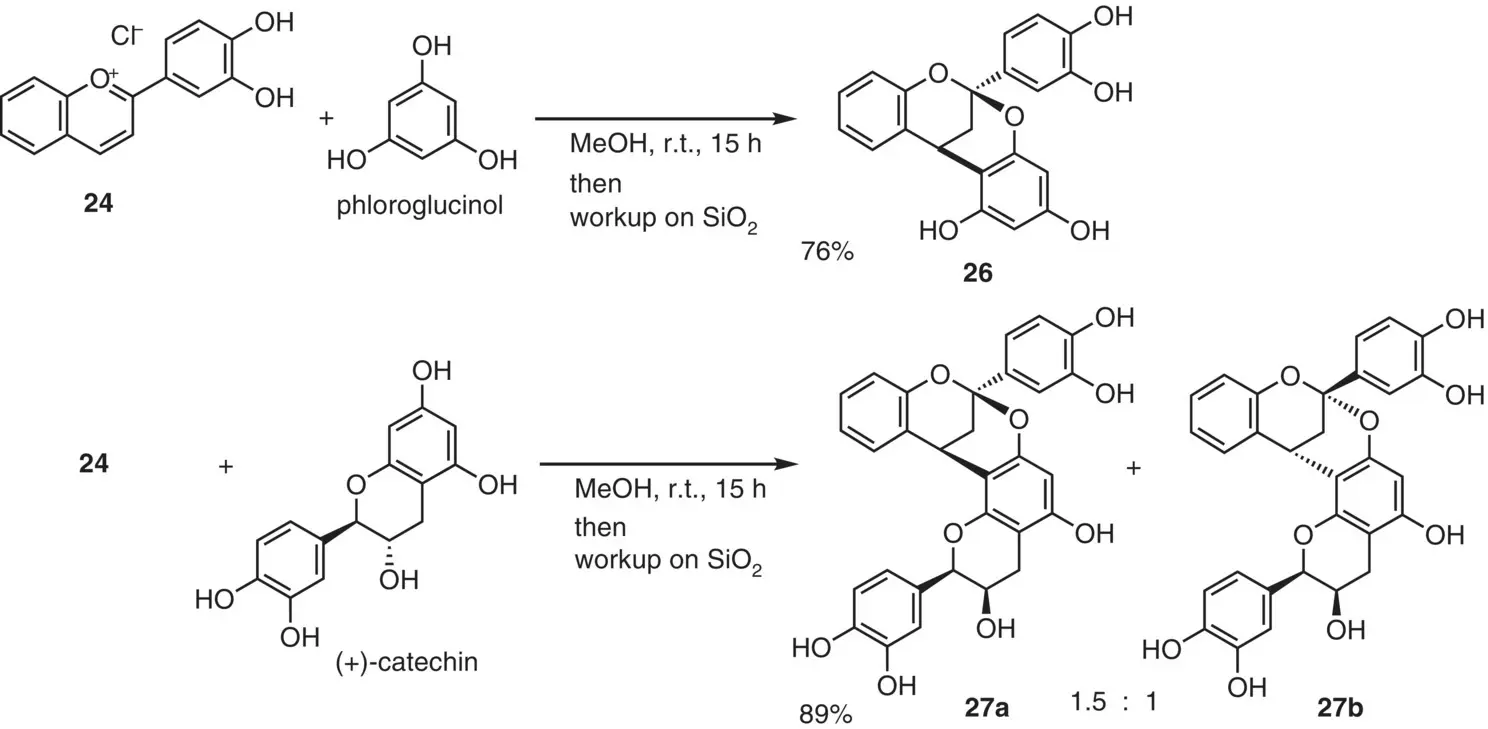
Figure 2.16 Annulation reaction by Kraus.
Читать дальше





















