Recent Advances in Polyphenol Research
Здесь есть возможность читать онлайн «Recent Advances in Polyphenol Research» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Recent Advances in Polyphenol Research
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Recent Advances in Polyphenol Research: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Recent Advances in Polyphenol Research»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Recent Advances in Polyphenol Research
Recent Advances in Polyphenol Research — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Recent Advances in Polyphenol Research», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
In the case of the 6,8 rearrangements for R=CH 3or phenyl (Basílio et al. 2017) the 8 derivative is the only one observed and equilibrium in moderately acidic medium is established between A 8and Ct(c. 20%). The pH‐dependent equilibrium between flavylium cation and a mixture of quinoidal base (major species) and trans ‐chalcone was previously reported for 5‐deoxyanthocyanidins (Sousa et al. 2014; Brouillard et al. 1982). At moderately acidic pH values there is some spectral evidence that the two quinoidal base isomers are in equilibrium, but the spectral variations are relatively small. In order to unveil the two isomers other strategies should be used; see below.
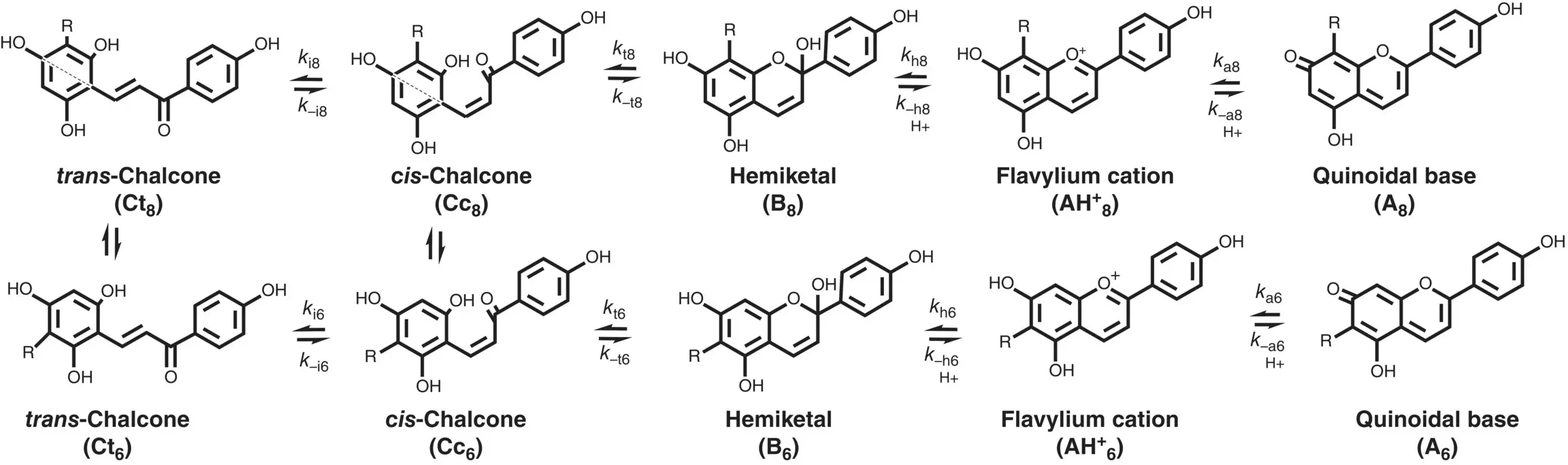
Scheme 1.13 General scheme of the 6,8 rearrangement. R=Br or R=CH 3or R=Phenyl.
Source: Adapted from Cruz et al. 2016. © 2016 John Wiley & Sons.
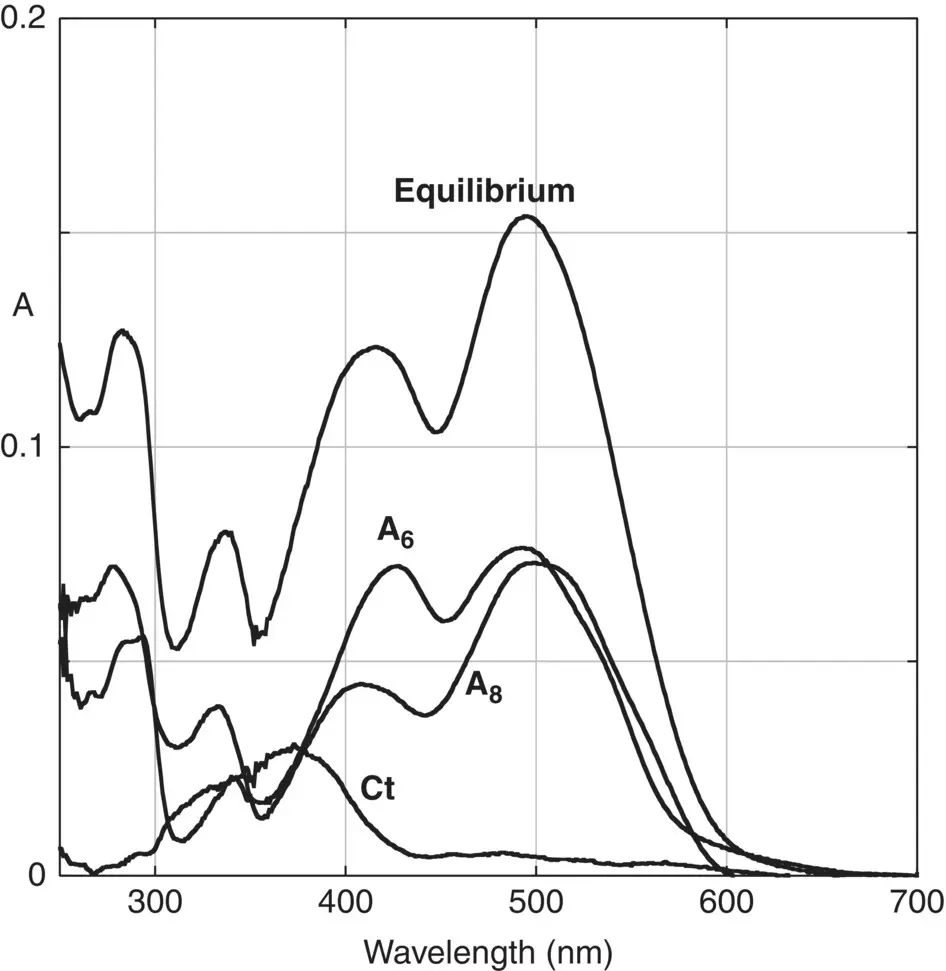
Figure 1.7 The absorption spectra of equilibrated solutions of the compound 8‐bromo,4′,5,7 trihydroxyflavylium R=Br (3.3x10 ‐5M) at pH=3.7 can be fitted with a contribution of 0.45 A 6+0.45 A 8+0.1 Ct.
Source: Adapted from Cruz et al. 2016. © 2016 John Wiley & Sons.
1.4.3.1 Unveiling the 6,8 Rearrangement Through Host‐Guest Complexation
A step further to the study of the 6,8 rearrangement was achieved by host‐guest complexation. It is known that while cucurbiturils complex preferentially the flavylium cation (Basílio and Pina 2014), cyclodextrins prefer the chalcones (Petrov 2013b). The sulfobutylether derivative of β ‐cyclodextrin (captisol) was used due to its better solubility in water, when compared with β ‐cyclodextrin. The complexation with captisol was used to unveil the 6‐phenyl isomer from the 8‐phenyl‐5,7‐dihydroxyflavylium (Basílio et al. 2017). The NMR and UV‐vis spectrophotometry indicates that the fraction of Ctincreases very significantly in the presence of captisol. Irradiation of this solution at pH=5.0 leads to a photostationary state, constituted by quinoidal bases A 6and A 8( Scheme 1.14). In fact, immediate acidification of the photostationary state to pH=1.0 shows the presence of the two respective flavylium cation isomers, with the 6‐phenyl isomer reverting slowly to the more stable 8‐phenyl isomer.
The host‐guest strategy was used to form the 6‐phenyl isomer from the 8‐phenyl‐5,7‐dihydroxyflavylium profiting from the capacity of cucurbit[7]uril to complex preferentially the former (Basílio et. al 2017) as well as for 6,8‐bromo‐apigeninidin (Basílio et al. 2016).
From bottom to top in Scheme 1.15, the 1H NMR spectra of the two isomers are shown. After addition of the host only the 6‐bromo isomer is observed. Addition of adamantine, which possesses a very high association constant with this host, permits to release and isolate the 6‐bromo isomer ( Scheme 1.16). The system slowly reaches equilibrium by formation of the 8‐bromo isomer.
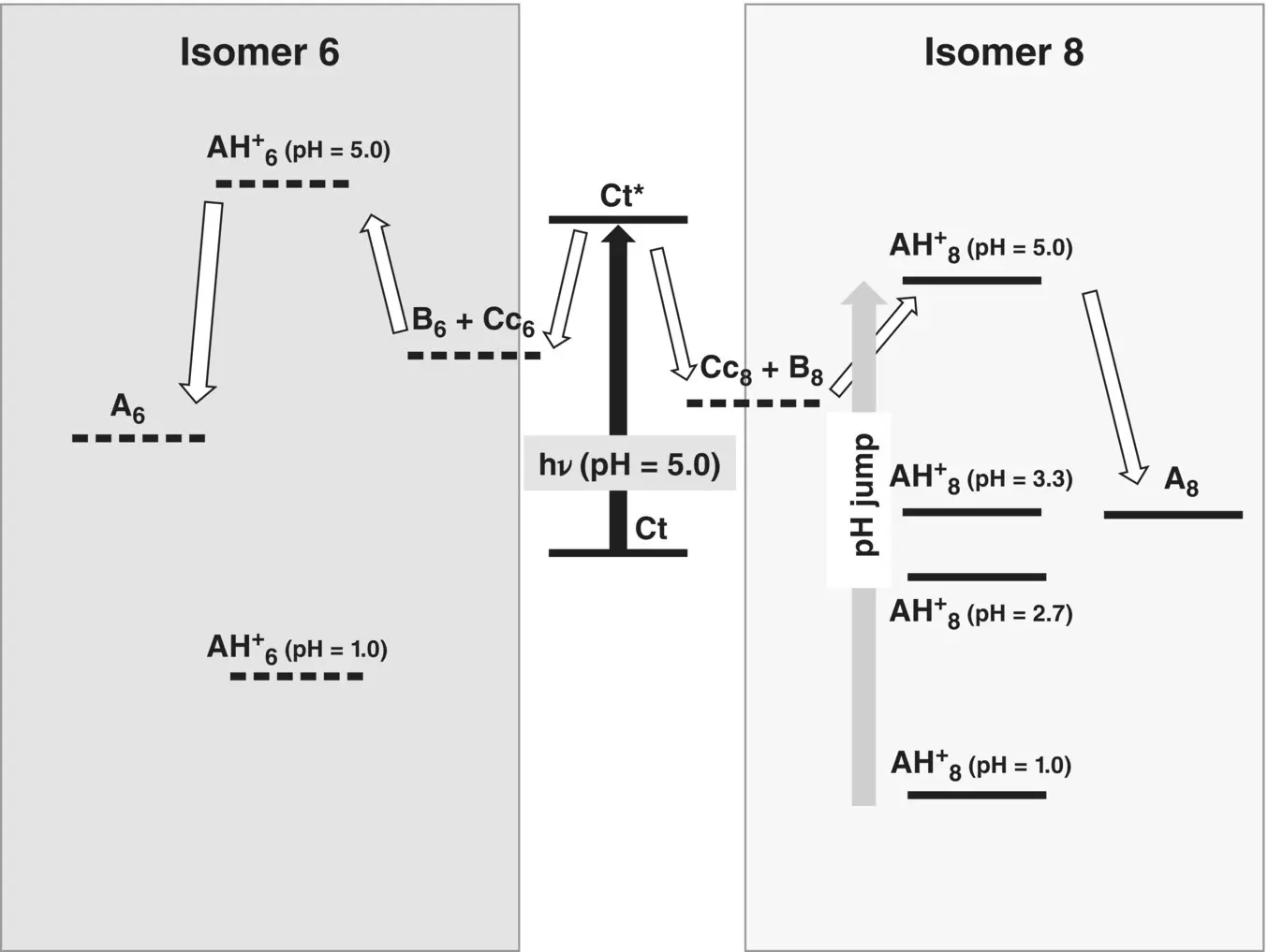
Scheme 1.14 Qualitative energy level diagram to account for the pH jumps and the photochemistry of the 6,8 rearrangement of 8‐phenyl‐5,7‐dihydroxyflavylium in the presence of the captisol host. Traced energy levels are tentatively positioned. The arrow of the excitation energy is not in scale.

Scheme 1.15 1H NMR of 6‐bromo‐5,7‐dihydroxyflavylium and its 8‐bromo isomer in the presence of cucurbit[7]uril.
Source: Adapted from Basílio et al. 2017. © 2017 John Wiley & Sons.
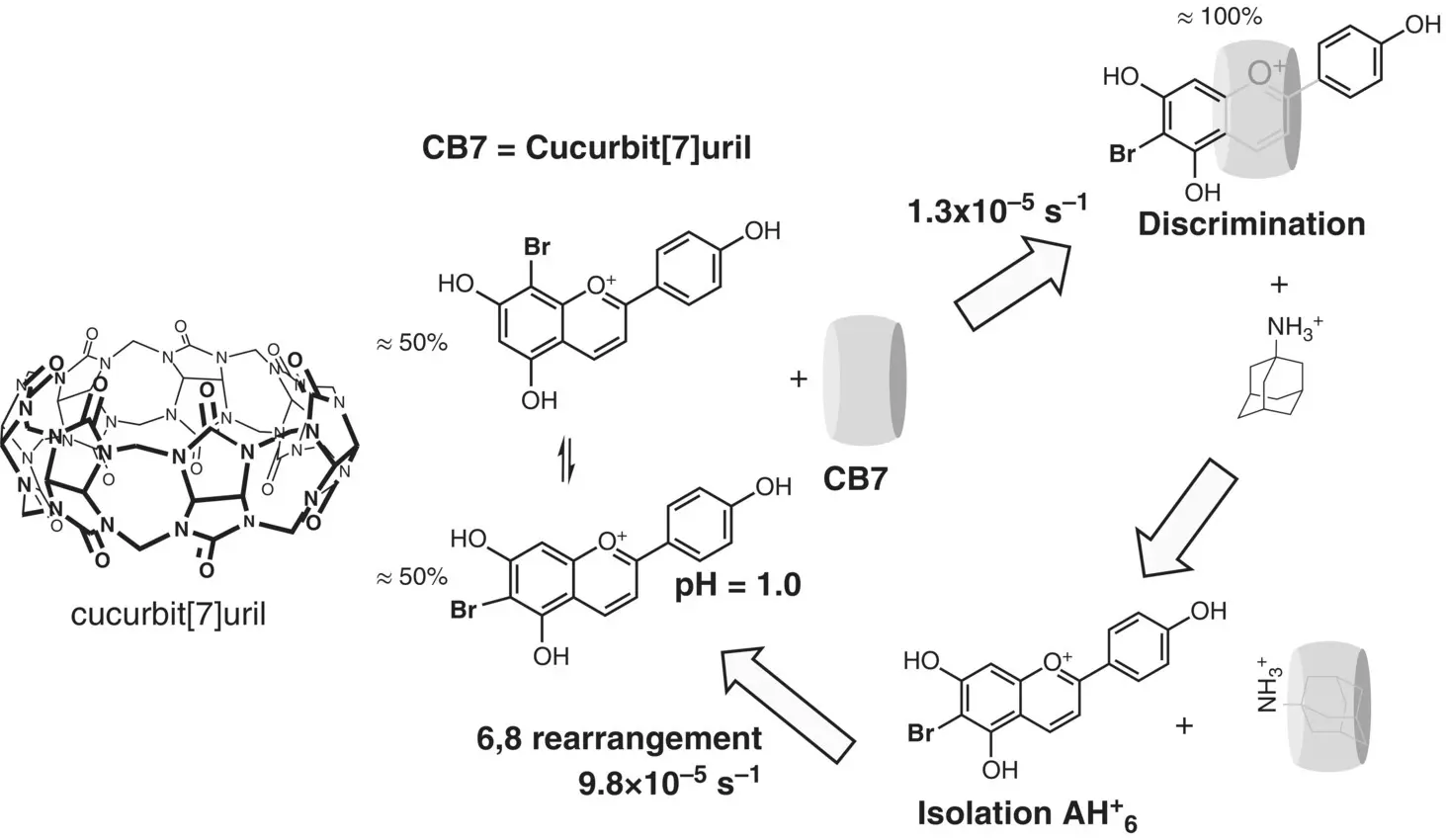
Scheme 1.16 Discrimination, isolation, and 6,8 rearrangement of 6‐bromo‐5,7 dihydroxyflavylium and its 8‐bromo isomer in the presence of cucurbit[7]uril. Reproduced from Basílio et al. (2017), with permission.
Source: Adapted from Basílio et al. 2017. © 2017 John Wiley & Sons.
1.5 Conclusions and Perspectives
The multistate of chemical species followed by anthocyanins is not restricted to this family of compounds. Besides those of natural origin such as anthocyanidins and deoxyanthocyanidins, anthocyanins have been a source of bio‐inspiration for the synthesis of new derivatives that permit us to achieve complexity at the bottom, through this multistate of chemical species. The multistate of chemical species can be operated by pH changes, light, temperature, and host‐guest chemistry. The light response is particularly challenging, since there are two photochromic systems: (i) the ring opening of the hemiketal to produce cis ‐chalcone, and (ii) cis‐trans isomerization of the chalcones. The multistate response can be modulated by host‐guest chemistry, increasing the potentialities of the system as photochromic materials, or in the domain of the health sciences.
References
1 Basílio, N., Cruz, L., Freitas, V., et al. (2016). A multistate molecular switch based on the 6,8 rearrangement in bromo‐apigeninidin operated with pH and host–guest inputs. Journal of Physical Chemistry, 120 (29): 7053–7061.
2 Basílio, N., Lima, J.C., Cruz, L., et al. (2017). Unveiling the 6,8 rearrangement in 8‐phenyl‐5,7‐dihydroxyflavylium and 8‐methyl‐5,7‐dihydroxyflavylium through host‐guest complexation. European Journal of Organic Chemistry, 37: 5617–5626.
3 Basílio, N., and Pina, F. (2014). Flavylium network of chemical reactions in confined media: modulation of 3’,4’,7‐trihydroxyflavilium reactions by host–guest interactions with cucurbit[7]uril. ChemPhysChem, 15 (11): 2295–2302.
4 Bjorøy, Ø., Rayyan, S., Fossen, T., et al. (2009). Structural properties of anthocyanins: rearrangement of c‐glycosyl‐3 deoxyanthocyanidins in acidic aqueous solutions. Journal of Agricultural and Food Chemistry, 57 (15): 6668–6677.
5 Brouillard, R., and Dubois, J.E. (1977). Mechanism of structural transformations of anthocyanins in acidic media. Journal of the American Chemical Society, 99 (5): 1359–1364.
6 Brouillard, R., Iacobucci, G.A., and Sweeny, J.G. (1982). Chemistry of anthocyanin pigments. 9. UV‐visible spectrophotometric determination of the acidity constants of apigeninidin and three related 3‐deoxyflavylium salts. Journal of the American Chemical Society, 104 (26): 7585–7590.
7 Cruz, L., Basílio, N., Freitas, V., et al. (2016). Extending the study of the 6,8 rearrangment in flavylium compounds to higher pH values. Inter‐conversion between 6‐bromo and 8‐bromo‐apigeninidinin. Chemistry Open, 5 (3): 236–246.
8 Sousa, J.L., Gomes, V., Mateus, N., et al. (2017). Synthesis and equilibrium multistate of new pyrano‐3‐deoxyanthocyanin‐type pigments in aqueous solutions. Tetrahedron, 73 (41): 6021–6030.
9 Cruz, L., Sousa, J.L.C., Gomes, V., et al. (2017). Chemical equilibrium characterization of pyrano‐3‐deoxyanthocyanin derivatives. Tetrahedron 73: 6012–6030.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Recent Advances in Polyphenol Research»
Представляем Вашему вниманию похожие книги на «Recent Advances in Polyphenol Research» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Recent Advances in Polyphenol Research» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












