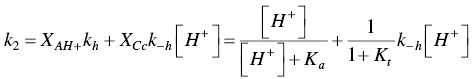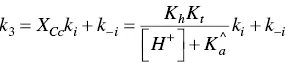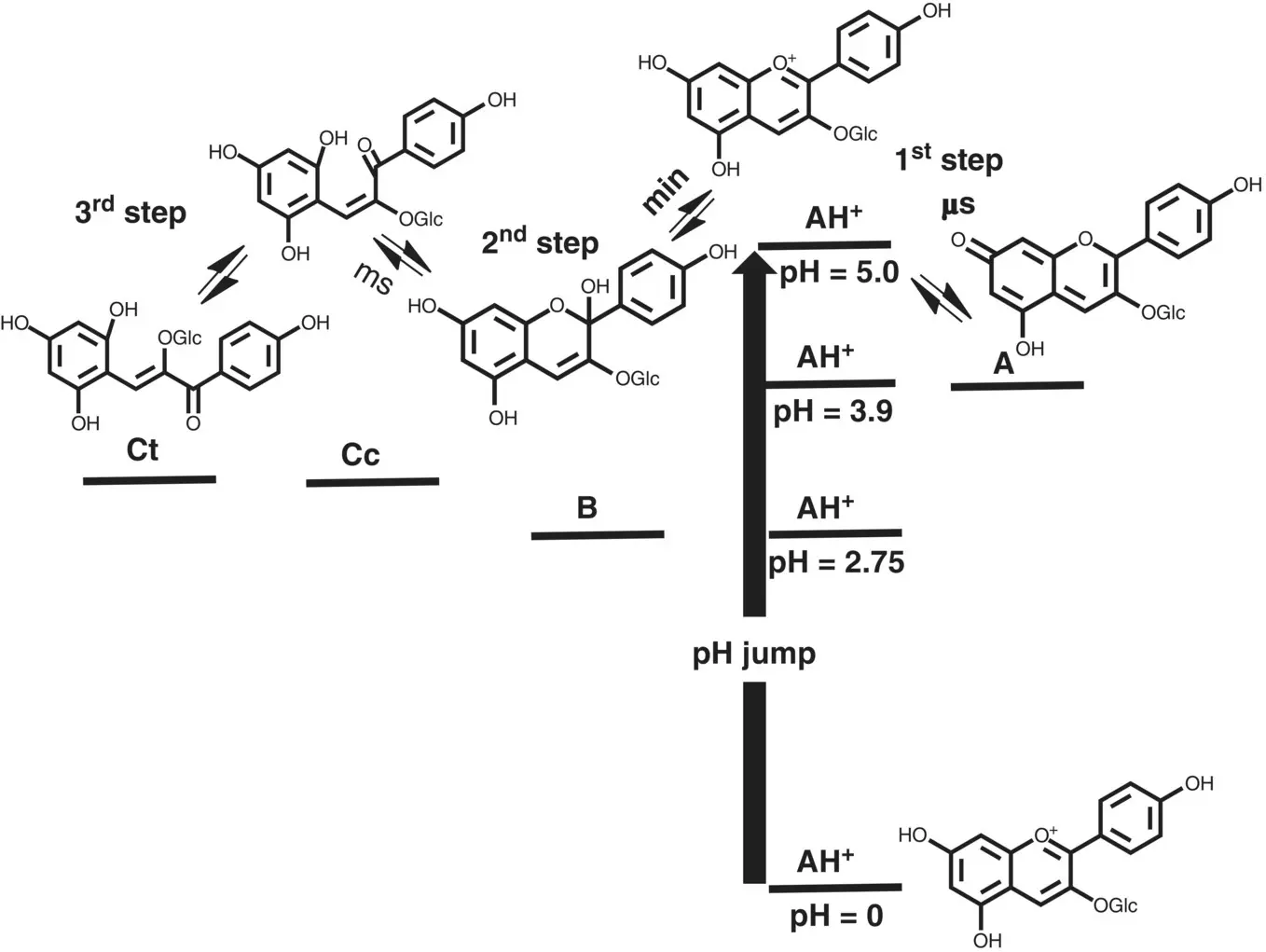
Scheme 1.5 Energy level diagram of the relative thermodynamic level of the five species of pelargonidin‐3‐glucoside appearing in acid medium. The three distinct kinetic steps taking place in very different time scales are observed, allowing for separation of the kinetics into three kinetic equations.
The following kinetic step is the hydration followed by tautomerization ( Scheme 1.5). Except in very acidic solutions (not accessed by direct pH jumps), the tautomerization reaction is faster than hydration and by consequence this last one is the rate‐determining step of this kinetic process. This kinetic step can thus be considered as in eq. (39).
During the hydration both AH +/ Aand B/ Cccan be considered as a single species.
(39) 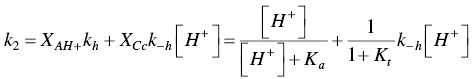
where X AH+is the mole fraction of AH +in its equilibrium with A, and X Bis the mole fraction of Ain its equilibrium with Cc.
In eq. ( 39)the forward reaction takes place only from the reaction of AH +to form B, because, as mentioned above, the quinoidal base Adoes not hydrate in acidic medium (Brouillard and Dubois 1977).
For anthocyanins and many of the flavylium derivatives, the last step is controlled by the isomerization of chalcones, which is by far the slowest process of the kinetics. A similar reasoning used for step 2 can be made for step 3. In this case all species except Ctcan be considered equilibrated.
(40) 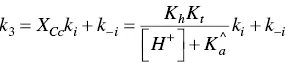
1.4.1 Heavenly Blue Anthocyanin
The experimental procedure above reported was used to rationalize the multistate of heavenly blue anthocyanin and two derivatives ( Scheme 1.6).
Heavenly blue anthocyanin, HBA1, has attracted the attention of the scientific community due to its peculiar properties, specifically the fact that the same anthocyanin is used by the plant to confer purplish color to the buds and blue color to the petals (Yoshida et al. 1995; Goto and Kondo 1991; Kondo et al. 1992). Moreover, in vitro the blue color is persistent in neutral and moderately basic solutions (Kondo et al. 1992; Yoshida et al. 2009). Structural information regarding HBA1 fully supports the intramolecular stacking shown in Scheme 1.7.
The system was studied up to the mono‐anionic forms because at higher pH values a slow decomposition takes place and the data does not have sufficient accuracy. In spite of equilibrium being reached in one to two weeks, the neutral and mono‐anionic species are relatively stable. Table 1.1summarizes the data.
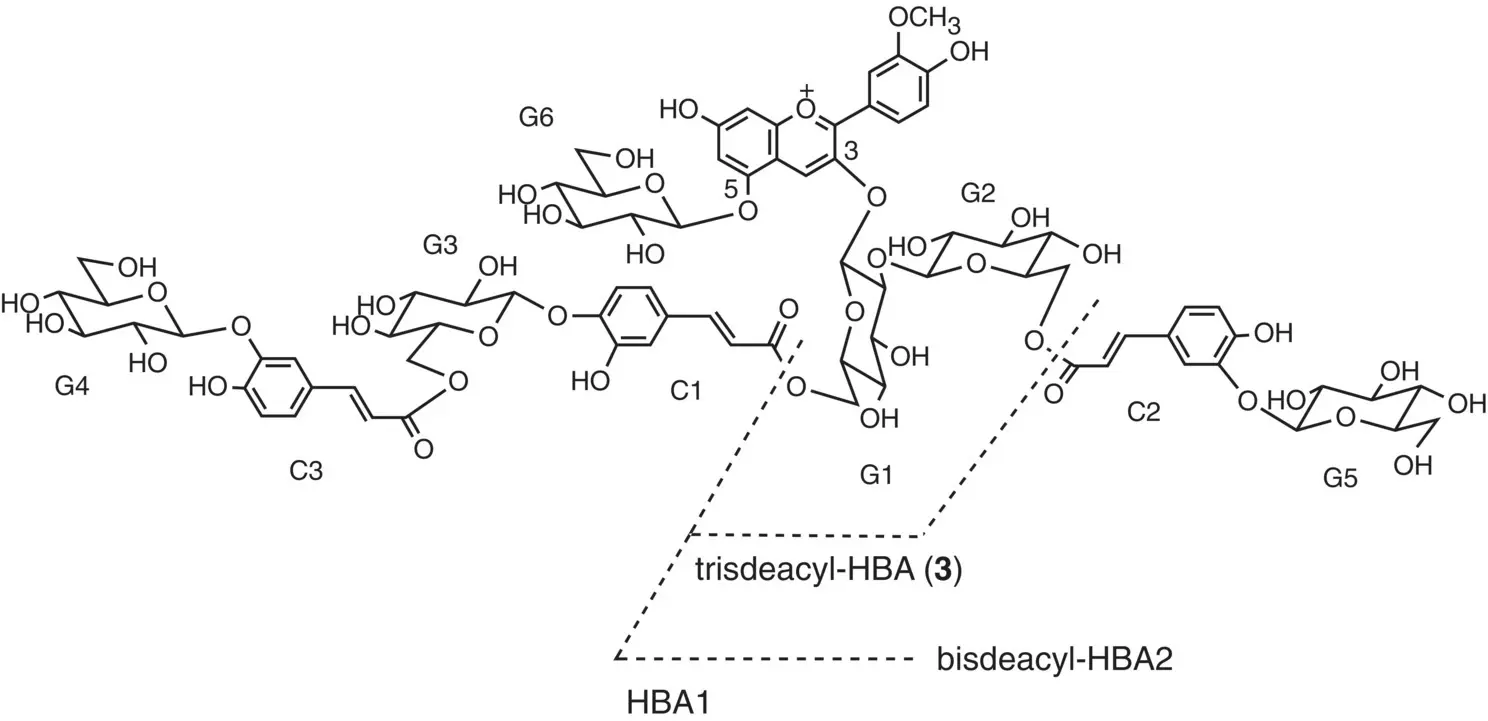
Scheme 1.6 Heavenly blue anthocyanin HBA1 and their derivatives bis‐deacyl‐HBA2 and tris‐deacyl‐HBA3.
Source: Mendoza et al. 2018.

Scheme 1.7 Sketch representing the intramolecular copigmentation in polyacylated anthocyanins; CPK models of heavenly blue anthocyanin.
Source: Mendoza et al. 2018.
Table 1.1 Equilibrium constants of heavenly blue anthocyanin and their derivatives.
Source: Mendoza et al. 2018.
|
p K ’ a |
p K ” a |
p K ^ a |
p Ka |
p K h |
K t |
| HBA1 |
3.5 |
7.3 |
3.6 |
3.8 |
4.6 |
1.1 |
| HBA2 |
— |
— |
2.92 |
4.23 |
3.1 |
0.35 |
| HBA3 |
— |
— |
1.95 |
4.19 |
2.1 |
0.37 |
|
K i |
pK ^^a |
pK A/A‐ |
pK B/B‐ |
pK Cc/Cc‐ |
pK Ct/Ct‐ |
| HBA1 |
4.0 |
7.35 |
7.35 |
7.5 |
7.25 |
7.36 |
Estimated error 10%.
In Table 1.1the equilibrium constants of the non‐acylated, di‐acylated and tri‐acylated derivatives of heavenly blue anthocyanin are also reported ( Scheme 1.8). HBA2 and HBA3 behave as common anthocyanins, being relatively stable only in acidic medium, preventing the calculation of the data regarding the anionic species at equilibrium.
The mole fraction distribution for HBA1 of the several species is represented in Figure 1.5. This distribution is in line with the previous observation (Yoshida et al. 1995) that the buds of heavenly blue anthocyanin are purple while the petals are blue. In fact the pH of the vacuoles in buds is around 6.6, while in petals pH=7.7 (Yoshida et al. 1995). In that pH region a small pH change gives different contributions of the quinoidal base and anionic quinoidal base and significant color changes can be observed.
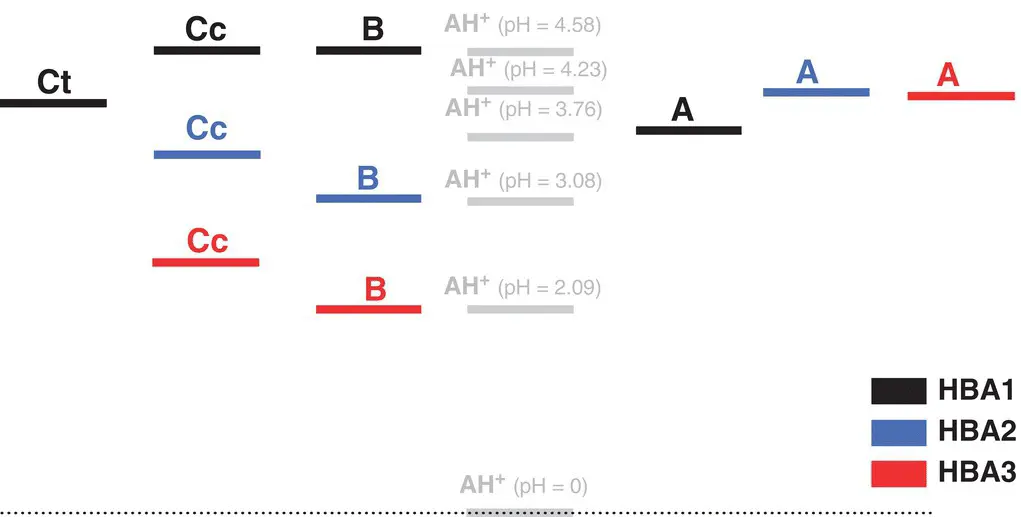
Scheme 1.8 Energy level diagrams of HBA 1(black), HBA 2(blue), and HBA 3(red). In HBA 1there is an inversion of the relative stability between the quinoidal base and hemiketal. The energy levels of Ctcannot be measured with the necessary accuracy due to some decomposition in HBA 2and HBA 3for longer reaction times.
Source: Mendoza et al. 2018.
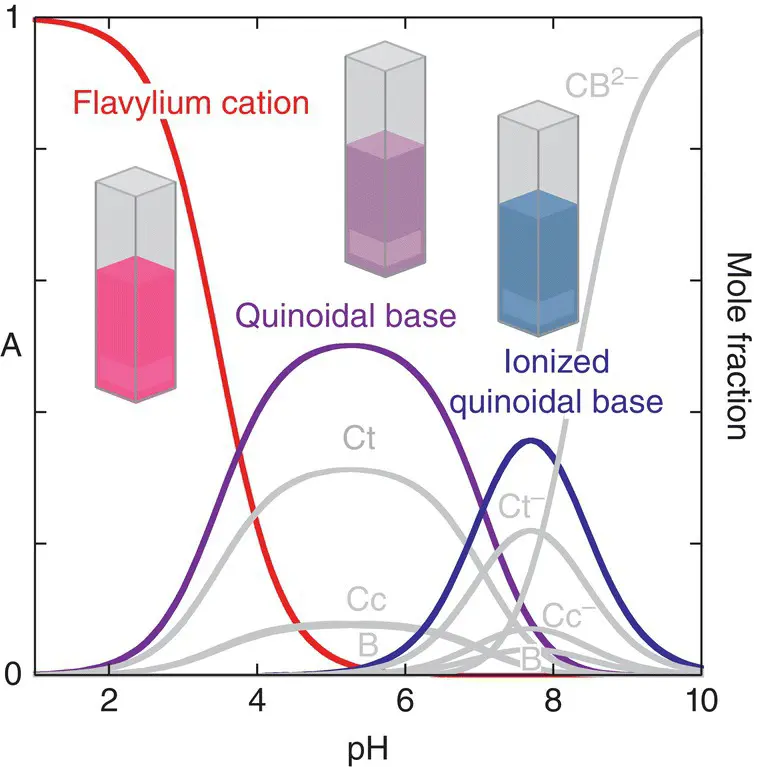
Figure 1.5 Mole fraction distribution of heavenly blue anthocyanin.
Source: Mendoza et al. 2018.
The rate constants of the kinetic steps of HBA1 and derivatives were also determined and are reported in Table 1.2, together with the data for HBA2 and HBA3. The isomerization is very slow and some decomposition was observed, preventing the determination of the isomerization constants with accuracy.
Inspection of Table 1.2shows that the hydration constant of HBA1 decreases 35‐fold when compared with HBA3, while the dehydration increases c. nine‐fold. This kinetic effect is compatible with the previous explanation considering a π‐π stacking effect of the caffeoyl residues that protect positions C2 and C4 from the water attack. This kinetic effect also has thermodynamic consequences, because the equilibrium constant K hincreases 306‐fold. In addition, the equilibrium constant of the quinoidal base does not change significantly. The most interesting feature in HBA1 is the inversion of the energy levels of the quinoidal base relative to the hemiketal ( Scheme 1.8).
Читать дальше