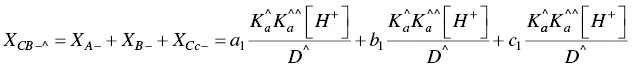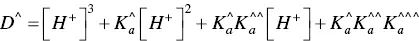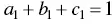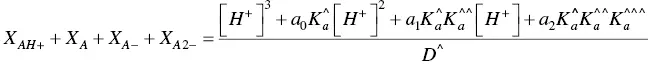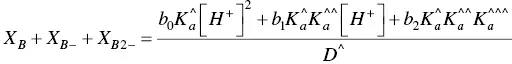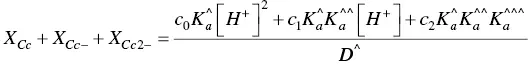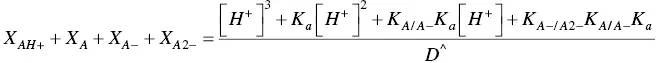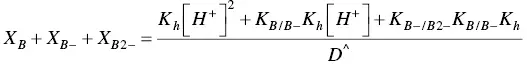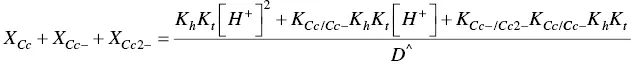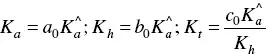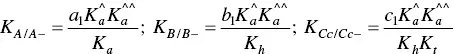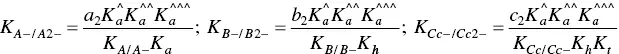In anthocyanins and most flavylium derivatives the cis‐trans isomerization is much slower than the other kinetic processes. It is possible thus to define a transient state (pseudo‐equilibrium) where the mole fraction of the trans ‐chalcones is very small. Consequently, it is more convenient to carry out the reverse pH jumps from pseudo‐equilibrium. Scheme 1.4illustrates the question in acidic medium, but it is generalized to higher pH values. Even if some Ctis formed, the only drawback is the loss of sensitivity, because the kinetics of the Cttransformation in flavylium cation is much slower and is not detected in the stopped flow experiments. In conclusion, the data reported in Figure 1.3, extended to other pH values, allows the calculation of the mole fraction distribution of the species A, B, and Ccas well as the respective anionic forms.
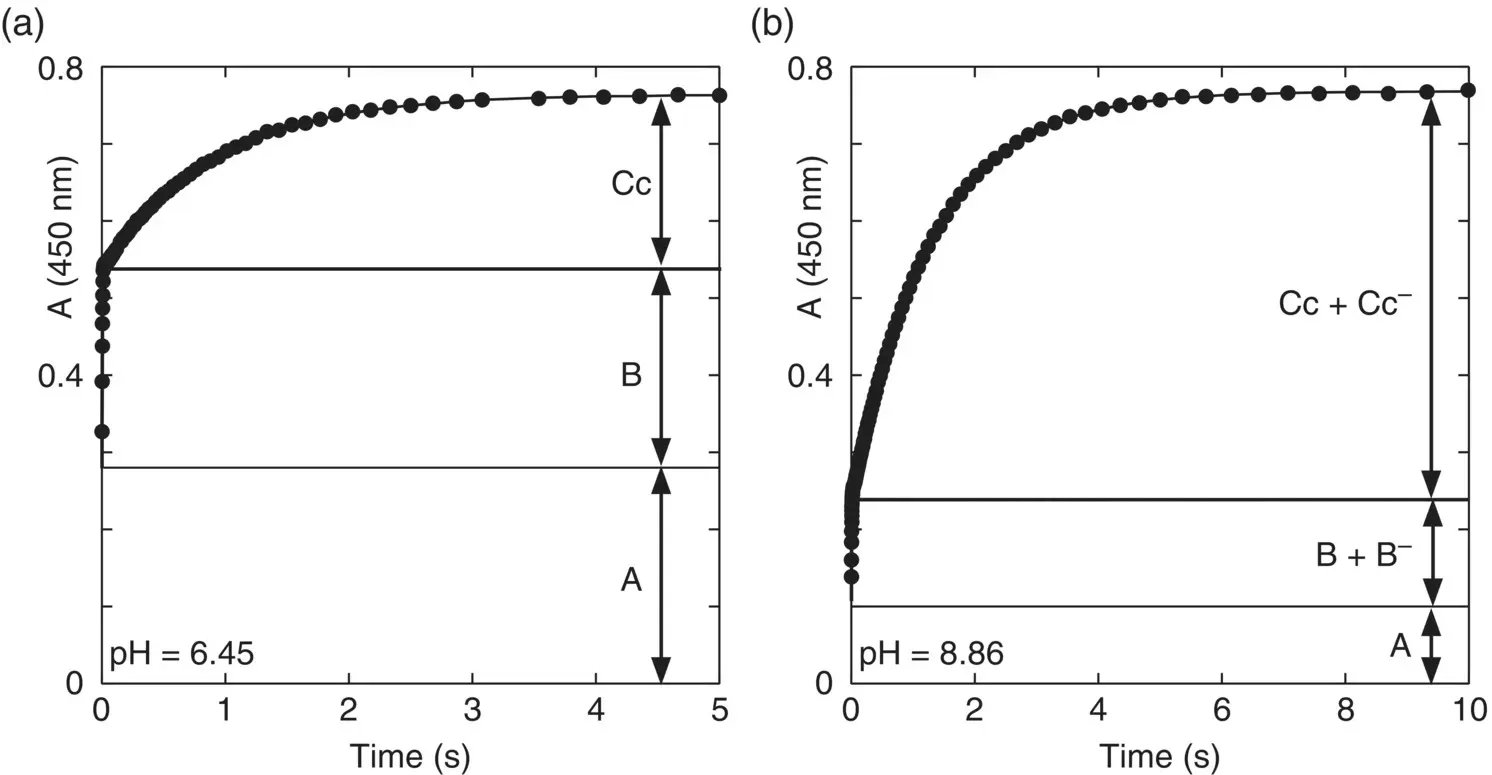
Figure 1.3 Stopped flow traces 4’‐hydroxyflavylium (at pseudo‐equilibrium, where no significant amounts of Ctwere formed) after a reverse pH jump from pH=6.45 (a) and pH=8.9 (b) to the final pH=1.0.
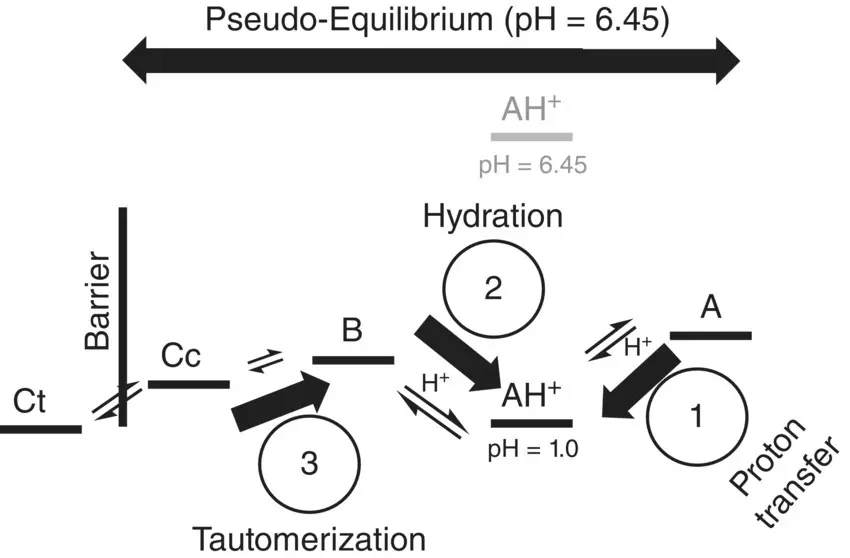
Scheme 1.4 Energy level diagram of the compound 4’‐hydroxyflavylium and the kinetic processes after a reverse pH jump to pH≤1.
The mole fraction distribution of these species can be represented as a function of the initial pH of the reverse pH jump ( Figure 1.4).
The fitting of Figure 1.4was carried out by considering for AH +, CB ^, CB ^‐, and CB ^2‐the contributions of the respective forms of quinoidal bases, hemiketals, and cis ‐chalcones. For example, the mole fraction distribution of CB ‐^is given by eq. (27)(Mendoza et al. 2019 supplementary information).
(27) 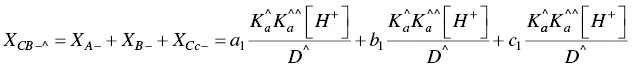
with
(28) 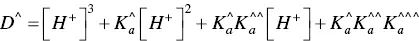
and
(29) 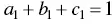
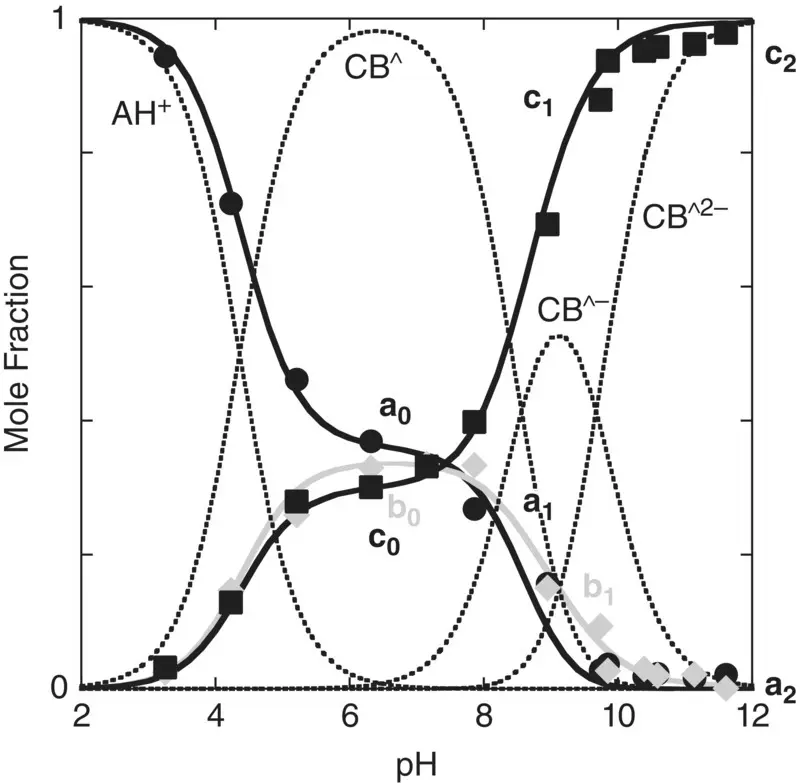
Figure 1.4 Representation of the mole fraction distribution of the compound 4’‐hydroxyflavylium on the basis that the reverse pH jumps at pseudo‐equilibrium. The symbol ^ is used to differentiate pseudo‐equilibrium from equilibrium (‘).
The mole fractions of the more colored forms, eq. (30), as well of those of hemiketals, eq. ( 31) and cis ‐chalcones, eq. (31) are thus obtained.
(30) 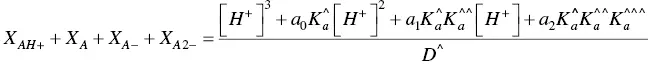
(31) 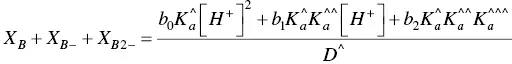
(32) 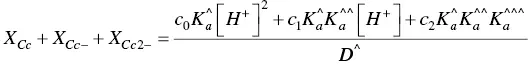
Considering that the apparent equilibrium constants are experimentally obtained from the inflection points of the absorption spectra as a function of pH, the fitting of eq. (30)to eq. (32)permits us to obtain the constants a n, b nand c n(n = 0, 1 and 2).
On the other hand, eq. ( 22)to eq. (25)can be re‐written for the pseudo‐equilibrium:
(33) 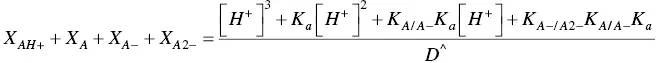
(34) 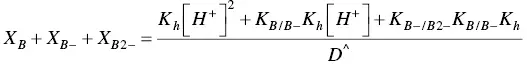
(35) 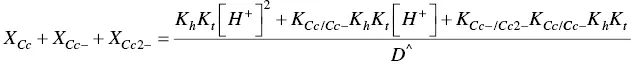
Comparing eq. (30)with eq. (33), eq. (31)with eq. (34), and eq. (32)with eq. (35)the following relations are obtained:
(36) 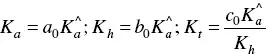
(37) 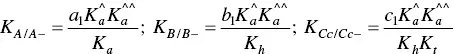
(38) 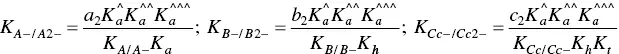
1.3.2 Reverse pH Jumps from Equilibrium
From eq. (36)to eq. (38)all equilibrium constants except those regarding the trans ‐chalcones can be obtained. Moreover, the cis‐trans isomerization constants can be calculated from a reverse pH jump from the equilibrated solutions. Considering that formation of the flavylium cation from the trans ‐chalcones is very slow, this kinetics should be followed by a standard spectrophotometer. The quinoidal bases, hemiketals, and cis ‐chalcones are transformed to flavylium cation much faster than the trans ‐chalcones and appear as an initial absorption. 2 From this point all the equilibrium constants have been calculated. The mole fraction of trans ‐chalcone is thus obtained from the ratio of the absorbance of the trace amplitude/total absorbance. The mole fractions of the other species at equilibrium are obtained from those at pseudo‐equilibrium, calculating the respective proportion. 3 For example, if at pseudo‐equilibrium A=0.3, B=0.2, and Cc=0.5 and the mole fraction of Ctat equilibrium is 0.5, the mole fractions of A, B, and Ccat equilibrium are the following: A=0.15, B=0.1, and Cc=0.25.
1.4 The Kinetic Processes
Scheme 1.5represents the four kinetic processes of anthocyanins and related compounds in acidic medium. It is worth noting that, like in the case of the formation of the quinoidal base from flavylium cation, all the other anionic species are formed as in Scheme 1.3, from proton transfer. This reaction represents step 1 in the kinetic process and takes place in microseconds during the mixing time of the stopped flow. Only using special techniques such as temperature jumps (Brouillard and Dubois 1977), and in some favourable cases flash photolysis, are these constants obtained. 4 This fact makes the kinetics reported in Scheme 1.5the only relevant ones upon direct pH jumps, since the formation of the anionic species is immediate when compared with hydration, tautomerization, and isomerization. Moreover, the first process after a direct pH jump (from flavylium cation) is the formation of the quinoidal base, which equilibrates with the flavylium cation. In the subsequent kinetic steps these two species behave as a single one.
Читать дальше