1.3.2 Forestry Residue and Crops
Herbal resources used for biomass energy production are in a wide range. Herbal sources include forest products, some tree species that grow very quickly, algae-like herbs, algae and energy plants grown in water. Energy sourced plants (C4) are panicum, pancitum, sugar beet, sweet millet, sweet sorghum, sugarcane, corn [36]. Ethyl alcohol and methyl alcohol are obtained from the biomass of plant origin. Energy plants absorb CO 2and use water better than other plants and become more resistant to drought. Ethanol is obtained from sugar carbonates (sugar cane, molasses and sorghum), starches (corn and potatoes) and cellulose plants (wood and agricultural residues) [37].
An average of 80-100 tons of wet or 25-30 tons of dry biomass is obtained from one hectare of field per year on a medium-yield land to produce biomass energy by making use of plant sources. It is certain that in semi-tropical regions, which are more suitable in terms of climatic conditions, the yield can reach 40 tons of biomass per hectare. The unit cost of the energy obtained from biomass can compete with other fuels [37, 38].
The main animal sources used to obtain the large-scale energy consist of the waste of cattle and sheep and poultry. Theoretically, pigs and cattle and poultry are considered to have the highest capacity to produce biomass energy. In general, it is observed that animal wastes are mixed with straw and dried, used as fuel and agricultural fertilizer [39]. Biogas produced by fermentation of animal manure in an oxygen-free environment is very common in the world [40].
Especially animal solid wastes are seen as the ideal source for biogas (65% methane, 35% CO 2) production after biological treatment. The biogas obtained provides benefits as an important energy input for both electricity and heat production. If the animal wastes are released into nature without being treated, important environmental health problems such as global warming caused by methane gas, groundwater contamination and pathogenic problems occur [40, 41].
The term industry wastes covers all kinds of domestic organic wastes and organic wastes that have been fabricated in industry. These wastes are easy to obtain and have almost unlimited supply. These wastes, which have been largely neglected in the past, can reach large amounts and cause important environmental problems [42]. The biomass obtained from these wastes can both eliminate environmental problems and provide resources for biofuel production. Another industrial waste is ash, which is also an important raw material for a variety of applications including building, agriculture and production of synthetic materials [43].
1.4 Methods of Conversion of Biomass to Liquid Biofuels
Technologies used to convert biomass into solid, liquid and gaseous fuels and valuable chemicals can be grouped under two main headings. These are hydrolysis conversion technologies, which includes biochemical methods and catalytic conversion, and thermochemical conversion technologies that process the decomposition of biomass by different methods. Biomass conversion technologies are shown in Figure 1.1.
Thermochemical conversion technology is based on the basis of converting biomass into products such as fuels and valuable chemicals with the effect of heat. The thermochemical conversion of biomass is one of the oldest processes used by humanity for purposes such as warming and cooking. Thermochemical conversion technologies include pyrolysis, liquefaction and gasification process [44].
Thermochemical conversion technology, which is based on obtaining thermal energy as a result of the rapid reaction of fuel with oxygen, is also a common and advanced technology to be used commercially as it is widely used in vehicles and factories. Gasification can be defined as the process of converting biomass into gaseous fuel or synthesis gas containing different amounts of CO and H 2[45]. In this process, a gasification medium such as steam, air or oxygen is needed. Oxygen is the most common active ingredient. The active ingredients interact with solid carbon and heavy hydrocarbons, turning them into lower molecular weight gases such as CO, H 2. With various conversion processes applied to biomass, solid, liquid and gas fuels (easily transportable, storable and usable) with high fuel quality, equivalent properties to existing fuels, and more useful, or valuable products for the chemical industry can be obtained. The variety of fuels derived from biomass varies depending on the conversion processes applied and the characteristics of the biomass used [46].
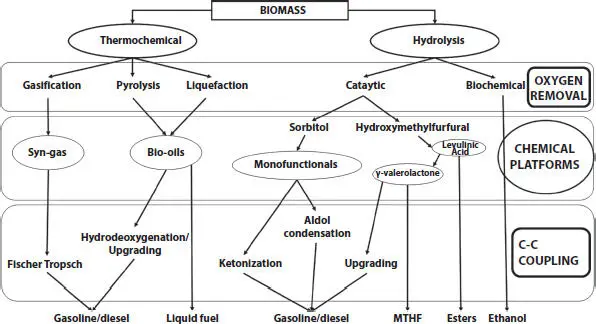
Figure 1.1 Strategies for conversion of biomass to liquid biofuels by thermochemical and hydrolysis routes.
1.4.1 Pyrolysis and Types of the Pyrolysis Processes
Pyrolysis is the most basic thermochemical conversion process used to convert biomass into more valuable fuel. Pyrolysis is defined as the heat degradation process of biomass in an oxygen-free environment. As a result of the pyrolysis process, hydrocarbon-rich gas, oil-like liquid and carbon-rich solid product are obtained. The amount of gas, liquid and solid products obtained as a result of pyrolysis depends on the applied pyrolysis method and operating conditions. Pyrolysis time and temperature are the most effective parameters on product yield and product variety. Various pyrolysis methods are applied depending on the product desired to be obtained. Pyrolysis processes can be grouped as “slow” and “fast” in general terms depending on the working conditions. Pyrolysis methods and conditions are given in Table 1.1. Solid, liquid and gaseous fuels are produced as a result of thermal degradation of the biomass in an environment free of air or any reactance. Thermal decomposition is called carbonization if the solid product yield is maximized. The main reaction from the pyrolysis of biomass is the separation of water from the carbohydrate compound as follows [47].
(1.1) 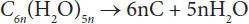
Table 1.1 Pyrolysis processes for different biomass.
| Biomass |
Catalyst |
Temperature (°C) |
Pressure (atm) |
Biochar yield (%) |
Biofuel yield (%) |
Reference |
| Mesue ferra seed oil |
Na 2CO 3 |
500-600 |
1 |
17.5 |
66 |
[49] |
| Canola oil |
HZSM-5 |
500 |
1 |
16.4 |
49.6 |
[50] |
| C. inophyllum |
KOH |
50 |
NA |
NA |
66.5 |
[51] |
| Rise husk |
KOH |
700 |
1 |
21 |
39 |
[52] |
| Waste clay oil |
CaO |
450 |
NA |
23 |
72 |
[53] |
| Rice straw & sugarcane |
HZMN-5 |
500 |
NA |
29.7 |
26.1 |
[54] |
| Microcrystalline cellulose |
NaY |
30-950 |
N 2atm |
19 |
48 |
[55] |
| Grape seeds |
CaO |
550 |
NA |
42.5 |
38.5 |
[56] |
| Ajjwa date seed |
Ni 3Fe |
500 |
10 bar H 2 |
15.8 |
58.1 |
[57] |
NA = not available
As a result of pyrolysis of lignocellulosic solid wastes, three main products are obtained. These are semi-coking solid product (char), oil and gas as seen in Figure 1.2. As a result of pyrolysis of cellulose, char and active cellulose are obtained, the products of active cellulose which are not used as a result of biofuel production which are separated. Wastes can be converted into solid, liquid and gaseous products with the pyrolysis process. The composition and proportions of these products largely depend on the type of raw material and reaction conditions. During pyrolysis of solid wastes, a wide variety and quite complex reactions occur in series and parallel. These complex reactions occur because the breakdown of cellulose, hemicellulose and lignin in the biomass content forms different products [48].
Читать дальше