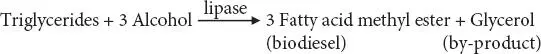1 ...7 8 9 11 12 13 ...24 Organic solvents also eliminate the need of stepwise addition of alcohol. All these things in combination increase production of biodiesel. Organic solvents that are commonly used include tert-butanol, petroleum ether, hexane, and n-heptane [102]. Some other organic solvents that are used are 2-butanol, cyclohexane, isooctane, acetone, 1,4-dioxane, and chloroform. While considering nature of organic solvents, hydrophobic organic solvents are majorly used. Hydrophobicity of the organic solvents helps in accumulating water molecules around enzyme which is important for enzyme structural stability [103]. Polar or hydrophilic solvents work opposite to hydrophobic organic solvents by playing role in distortion of enzymatic structure. But solvent with little polarity can be beneficial to dissolve oil and alcohol. For example, hydrophilic 1,4-dioxane and tert-butanol have produced some good results by producing high enzymatic transesterification yield [104]. Tert-butanol, having moderate polarity, eliminates glycerol and methanol inhibition problem for enzyme because it can dissolve both in itself. This makes the enzyme more stable and active and then ultimately produce better reaction yield [105]. Tertbutanol is the most common solvent that proved its effectiveness in various cases. According to Royon et al . [84], cottonseed oil was transesterified in the presence of Candida antartica lipase. Methanol was found to be the cause of enzyme inhibition in the reaction but when tert-butanol was used as solvent, reaction yield goes up to 97% with minimal enzyme inhibition. Similarly, in another research experiment, tert-butanol was tested for its effectiveness when rapeseed oil was used as substrate for biodiesel production. In solvent-free system, methyl ester yield was 10% but after utilizing tert-butanol yield was 75%. But under optimum conditions having Lipozyme TL IM and Novozyme 435 both in the reaction system, biodiesel yield reached 95% and the reaction was so stable that enzymes did not lose their activity even after 200 cycles. Reaction was favored and well supported by tert-butanol [86].
Use of solvents provide many benefits but they also come with some disadvantages such as organic solvents do not completely dissolve glycerol, by-product of the reaction, that causes the enzyme to lose its activity and become unstable. Use of solvents also make the process very costly because there is a need of extra purification step to separate out solvent and product from the reaction mixture. Organic solvents are mostly toxic and highly flammable so there are also environmental and health concerns while using them [11]. In order to tackle problems of conventional organic solvents, researchers have suggested some alternatives. Diesel oil was found to be an interesting alternative but the most recent, beneficial, and popular alternatives are super critical carbon dioxide (SC-CO 2) and ionic liquids (ILs). Researchers have also confirmed the positive effect of using SC-CO 2and ILs in the enzymatic transesterification [106, 107].
1.7 Lipases as Biocatalysts for Biodiesel Production
Transesterification of oils for biodiesel production is done using either chemical or enzymatic catalyst [108]. An enzymatic catalyst is used at first place due to their normal reaction conditions, reusability, easy products separation, and production of high-quality product. There is less energy consumption in enzyme catalysis as it occurs at a low temperature as compared to chemical catalysis requiring high energy consumption [109, 110]. Further, enzymatic catalysis is environment-friendly as there is no wastewater production and produces pure biodiesel as compared to chemical catalysis [107]. Among enzymatic catalysts, lipase with excellent biochemical and physiological properties is most commonly used to catalyze the transesterification process. Lipases play their role in several industrial processes like alcoholysis, acidolysis, amynolysis, and hydrolysis reactions but their leading role in biodiesel production is considered very important [108–111]. The use of lipase in biodiesel production is proved to be beneficial due to its characteristics like high efficiency, convert FFAs completely into methyl/ethyl esters, reaction specificity, require low temperature, minimum energy consumption, and fewer side products [109]. Lipases belong to class “hydrolases” as they carry out hydrolyses of triglycerides producing glycerol and fatty acids from it in an oil-water interface [110]. A general reaction for biodiesel production using lipase is as follows:
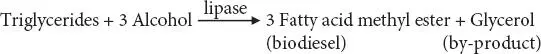
Lipases work on specific substrates and carry out catalysis of heterogeneous reactions in water-soluble as well as insoluble systems. Further, lipases have the properties like chemo-specificity, region-specificity, and stereo-specificity [111]. When classification is made based on region-specificity, there come three classes of lipases: 1) non-specific lipases, 2) 1,3-specific lipases, and 3) fatty acid-specific lipases. Non-specific lipases have ability to attach with all the possible positions of triglycerides to give FFAs and glycerol. The intermediates of the reaction, diglycerides, and monoglycerides do not accumulate in the reaction as they are instantly hydrolysed into fatty acids and glycerol [112]. 1,3-specific lipases are specific for the 1 and 3 positions of triglycerides and remove fatty acids from these positions. 1,3-specific lipases carry out the conversion of triglyceride to diglycerides much faster than diglyceride to monoglyceride [113]. Fatty acid-specific lipases carry out hydrolysis of a specific type of esters which have double bonded long chains of fatty acids in cis position between C-9 and C-1. Hydrolysis of esters with unsaturated fatty acids occur slowly and such class of lipases is not much common [114]. All the hydrolytic enzymes including lipases have common folding pattern involve in a hydrolytic activity called α/β hydrolase fold which is made up of a β sheet of eight strands (one of which is antiparallel while remaining seven strands are parallel) connected by α helices. Histidine residue, catalytic acid residue and Nucleophilic residue are present in α/β hydrolase fold. Pentapeptide sequence (Gly-X-Ser-X-Gly) which is a highly conserved in most of the lipases involved in the construction of ‘nucleophilic elbow’ which is a typical β-turn-α motif having active nucleophilic serine residue between a β strand and an α-helix. Catalytic triad made up of amino acids like histidine, serine, and aspartic acid or glutamic acid build the active site of lipases. The same catalytic triad is seen in serine proteases predicting common catalytic mechanism in them. Amphiphilic α helix peptide sequence forms a lid or flap which covers the active site of lipase and has a structural variability depending upon the lipase source organism. Changes in the structure of the lid are responsible for the activation/inactivation of lipases [114]. Changes in the conformation of lipase structure as well as the quality and quantity of interface being used in the reaction are responsible for the activation of lipase. When the lipase enzyme meets the oil/water interface there occur some changes in lipase structure that results in its activation. For the activation of lipase first, the lid opens to uncover the active site of lipase upon its contact with the ordered interface [115]. Due to this restructuring of lipase, electrophilic region is created around serine residue present in active site, lid hydrophilic side which was exposed in native form now partly buried inside the polar cavity and hydrophobic side of lid completely exposed, thus creating a non-polar surface around the active site for efficient attachment of lipid interface with it [115].
Читать дальше