Speers et al . (2014) studied anodic EF of glycerol with a customized co-culture of electro-active Geobacter sulfurreducens and glycerol-fermenting Clostridium cellobioparum . For industrially relevant loadings of glycerol, the system was able to provide ethanol concentration up to 10 g/L with 90% yield (glycerol-to-ethanol). In their dual-chamber system, anodic Geobacter sulfurreducens played a key role in this process by utilizing by-products of glycerol fermentation for the current generation. The electrons transferred to the anode were utilized for electrochemical hydrogen production on the cathode, which could provide an added-value-product from the process.
In another study, Awate et al . (2017) investigated EF of cellobiose with a bioanode comprised of cellulose-degrading fermentative bacteria Cellulomonas uda and a genetically engineered Geobacter sulfurreducens . In their single chamber reactor, Cellulomonas uda efficiently hydrolyzed and fermented cellobiose to ethanol, while Geobacter sulfurreducens rapidly utilized non-ethanol by-products and prevented feedback inhibition of ethanol fermentation. The electron captured by Geobacter sulfurreducens then converted to hydrogen gas on the cathode. Furthermore, genetic modification of Geobacter sulfurreducens provided improve lactate (a fermentation by-product) oxidation, while preventing the oxidation of hydrogen produced on the cathode. Overall, their single-chamber EF demonstrated higher ethanol concentration (0.332 vs. 1.226 g/L), yield (0.103 vs. 0.275 g/g cellobiose), and productivity (0.015 vs. 0.056 g/L/h) over conventional fermentation. Studies by Awate et al . (2017) and Speers et al . (2014) suggested that anodic EF with designer bioanode combing electroactive and fermentative bacteria could efficiently remove fermentation by-products in both single and dual-chamber systems (see Figure 1.4). Thus, EF can potentially reduce downstream purification costs, which can be as high as 80% of the total cost (Schievano et al ., 2016).
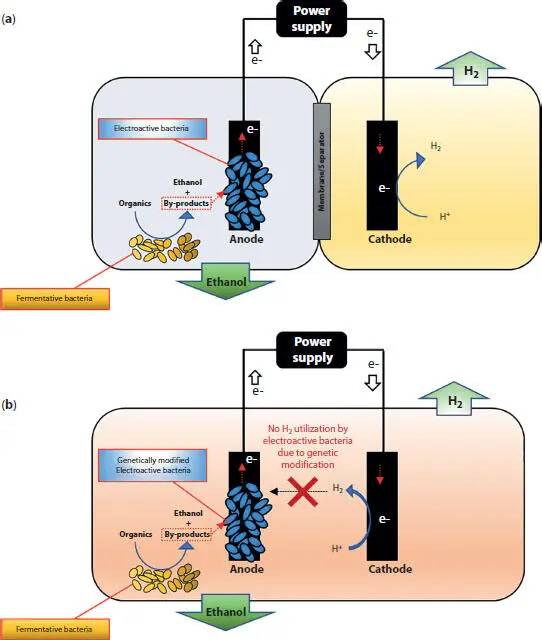
Figure 1.4 A conceptual schematic showing anodic ethanol-fermentation using customized co-culture of fermentative and electroactive bacteria using (a) dual-chamber, (b) single-chamber electro-fermentation systems. The figures draw on concepts proposed by Awate et al . (2017) and Speers et al . (2014).
Chandrasekhar et al . (2015) demonstrated a solid-state EF for simultaneous biohydrogen and bioethanol generation from food waste. The authors reported a maximum hydrogen production rate of 21.9 ml/h and maximum ethanol production of 4.85% (w/v). Although the authors did not compare the performance with a control reactor, their study demonstrated the possibility of valorization of real organic waste (i.e., food waste) for bioethanol production via EF. As shown in Table 1.1, the majority of EF studies were conducted with synthetic substrates. Therefore, future EF should consider utilizing real waste biomass to demonstrate the feasibility of EF for practical application.
Bio-butanol is another increasingly popular platform chemical that can also be used as liquid biofuel or additive to fossil fuel due to high energy content (27.08 MJ/l) and compatibility with combustion engines (Kumar and Gayen, 2011). The fermentation process driven by solventogenic Clostridia has been widely used for biobutanol production from carbohydrates-rich feedstock due to its low capital cost compared to the petrochemical butanol production process (Elbeshbishy et al ., 2015). Before 2005, butanol was mainly used for the synthesis of other industrial chemicals. After being recognized as a biofuel, the demand for butanol has considerably increased in the last 15 years (Kumar and Gayen, 2011). The global market of biobutanol is now expected to reach USD 17.78 billion by 2022 (Grand View Research, 2020).
Low biobutanol yield, as well as production rate, have been identified as major bottlenecks for wide-scale application of fermentative biobutanol production (Elbeshbishy et al ., 2015). Over the years, various strategies, including the deployment of genetically modified Clostridia and non-Clostridia organisms as well as designing novel fermentation systems, have been considered for alleviating these bottlenecks. In recent years, a few studies investigated biobutanol production via EF of glucose with pure-culture Clostridium species (Choi et al ., 2014; Engel et al ., 2019; Mostafazadeh et al ., 2016). Choi et al . (2014) studied cathodic EF of glucose with pure culture Clostridium pasteurianum DSM 525 in a dual-chamber EF system. The authors found that C. pasteurianum could produce butanol by utilizing electrons from both cathode and substrate (glucose). Although NADH generation from electricity was trivial as compared to that generated from glucose, EF could increase butanol production by 2.5 times over the control fermenter. Thus, their results suggested a metabolic shift in reduction pathways in C. pasteurianum due to the applied potential.
A study by Mostafazadeh et al . (2016) also reported enhanced biobutanol yield (g butanol/g consumed glucose) and productivity (g butanol/L-h) in cathodic EF using pure-culture C. pasteurianum . Compared to the control (i.e., conventional fermentation), butanol yield and productivity increased by 29% and 30%, respectively. Furthermore, their study highlighted the importance of process optimization of EF for biobutanol production. The authors investigated the effects of various process parameters, including glucose concentration (86.3-153.6 g/L), temperature (27.6-39.4°C), and applied voltage (0-2.6 V) using statistical design of experiment in cathodic EF of glucose to biobutanol using pure-culture C. pasteurianum . The maximum biobutanol concentration of 13.31 g/L butanol was achieved at an applied voltage of 1.32 V under an operating temperature of 33.51°C and at a glucose concentration of 120 g/L. The glucose concentration ≥140 g/L introduced inhibition and led to a decrease in butanol yield. Furthermore, the authors also compared the impact of cathode electrode materials on butanol production. The results showed that up to an applied voltage of 1.5, graphite felt as cathode could provide higher butanol concentration as compared to the stainless-steel electrode. However, with the applied voltage >1.5 V, the performance of both electrodes declined. With an increase in applied voltages, hydrogen production on the cathode linearly increased in both reactors. Moreover, the stainless-steel electrode led to higher hydrogen production as compared to the graphite, which is consistent with some of the reports on superior hydrogen evolution reaction (HER) with the metal electrode (Cheng et al ., 2009). Thus, superior HER on the stainless surface could introduce instability of cathode biofilms. Overall, this study demonstrated considerable scope to optimize process parameters and design of EF systems to maximize productivity and yield of target value-added products.
1.3.4 Microalgae Derived Lipids
Up to date, fossil fuels have been widely adopted as energy sources across the world. However, there is a great need to significantly develop the renewable fuels (e.g., non-fossil fuel) as energy sources on a large scale to outcompete the fossil fuels, eliminating the issues of aggravating CO 2concentrations and global warming (IPCC, 2018; Liu et al ., 2019; Liu et al ., 2020c). Out of many renewable fuels, microalgae-derived biofuels have demonstrated to be highly promising due to their advantages, such as high biomass production per unit area, a high weight ratio of lipids and ability to accumulate lipids in oleosomes, and no competition for arable land usage (Adeniyi et al ., 2018; Chisti, 2007; Hallenbeck et al ., 2016; Hu et al ., 2008; Rittmann, 2008). Despite these attracting features, several drawbacks for the microalgae biofuel generation have been reported, emphasizing their high costs, and environmental concerns associated with algae harvesting and lipids extraction (Markou and Nerantzis, 2013; Pierobon et al ., 2018; Rittmann, 2008; Sills et al ., 2013). For instance, the requirement of pre-treatment (e.g., acid/alkaline hydrolysis, pulsed electric fields, ultrasound (Sheng et al ., 2011; Zbinden et al ., 2013)) for microalgae processing are generally highly energy intensive (e.g., in terms of both capital and operational costs), which was one of the arguments against further development and scaling-up. Lipid extraction is a critical step prior to the microalgal biodiesel production process. However, highly toxic chloroform-methanol solvents have been widely adopted for lipid extractions due to their effective performance (e.g., the ability to penetrate cell wall and membrane) (Bligh and Dyer, 1959; Folch et al ., 1957). However, they must be replaced by other non-toxic solvents (e.g., hexane-isopropanol) due to the environmental and public health concerns (Lai et al ., 2016a; Lai et al ., 2014). Hence, a greener approach is required to address the challenges associated with microalgae-derived lipids extraction.
Читать дальше













