Figure 1.1 Overview of various value-added products produced via electro-fermentation.
1.2 Fundamental Mechanisms
A system for EF consists of an anode and a cathode, and the chambers can be separated by an ion-exchange membrane (see Figure 1.2). The use of a membrane is optional; used when preventing product crossover is critical. Briefly describing the entire process, the EF comprises the fermentation of an energy-rich substrate, where the solid electrodes present in the EF system serves as inexhaustible electron donors or acceptors that does not limit the entire fermentation process (Jiang et al ., 2019; Moscoviz et al ., 2016). The EF system is generally connected with power sources (e.g., power supply, potentiostat, etc.), where the externally poised potential/voltage is utilized to regulate the fermentation pathways for pure and mixed cultures (Jiang et al ., 2019; Moscoviz et al ., 2016; Schievano et al ., 2016). Briefly speaking, the electrons are transferred between the fermentation medium and the bacteria (e.g., fermentative and/or electroactive), and between the bacteria and the electrodes (e.g., anode or cathode). The electron transfer process between bacteria and electrodes are known as extracellular electron transfer (EET). Depending on the type of fermentation, the EET can be outward (during anodic EF) and inward (during cathodic EF) (Gong et al ., 2020; Kracke et al ., 2018). Although EET mechanisms have not been studied exclusively for various EF processes, literature suggests that bi-directional EET can occur via multiple mechanisms, including direct electron transport via extracellular redox co-factors (e.g., cytochromes, and other redox proteins), nanowires, and mediators (Gong et al ., 2020; Kracke et al ., 2018).
Depending on the target product (e.g., final product more oxidized vs. reduced form than the initial substrate), the EF can be classified into two main types: (i) anodic electro-fermentation (AEF); and (ii) cathodic electro-fermentation (CEF). When the final product of EF is more oxidized form than the substrate (e.g., ethanol from glycerol), the working electrode acts as an anode and is used to dissipate the excess electrons, known as the AEF. On the other hand, if the final product is in a more reduced form than the substrate (e.g., butanol from glucose), then the working electrode acts as an electron donor, known as the CEF. The electron sinks during AEF are known to synthesize more adenisine triphospates (ATPs) through creating a proton gradient, while the electron sources during CEF have impacts on the generation of more reduced redox cofactors, such as NADH (Kracke and Krömer, 2014). Hence, both AEF and CEF can significantly enhance the entire fermentation performance (e.g., product selectivity, production rate and yield) (Xafenias et al ., 2017). Detail descriptions of underlying mechanisms or EF mechanisms can be found elsewhere (Gong et al ., 2020; Jiang et al ., 2019; Moscoviz et al ., 2016).
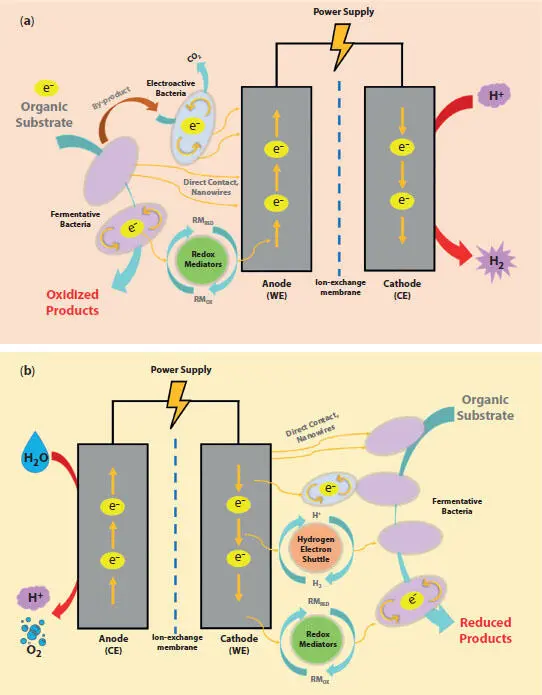
Figure 1.2 Mechanisms of electro-fermentation: (a) anodic electro-fermentation; (b) cathodic electro-fermentation.
As discussed earlier, in most cases, the reactions and electron transfers associated with EF are usually performed via syntrophic interactions between the fermentative bacteria and electroactive bacteria (Jiang et al ., 2019; Moscoviz et al ., 2016). However, sometimes, none of the fermentative bacteria are electroactive (e.g., Clostridium species), in which, the redox mediators, such as neutral red (Choi et al ., 2012), methyl viologen (Kim and Kim, 1988), or ferricyanide (Xafenias et al ., 2017) are required during the fermentation to impact the extracellular ORP (Choi et al ., 2012; Kim and Kim, 1988; Sturm-Richter et al ., 2015). When the redox mediators are introduced, they can first, be oxidized or reduced by the fermentative bacteria, then they are recycled or recovered electrochemically by the anode or cathode electrodes (Moscoviz et al ., 2016). In this context, the redox mediators are used as electron shuttles, and this process is known as the mediated electron transfer (Gong et al ., 2020; Rabaey and Rozendal, 2010; Thrash and Coates, 2008). Furthermore, other studies demonstrated another way to add a redox mediator in CEF, such as using produced H 2at the cathode that can be further used as a one-way electron shuttle (Gong et al ., 2020; Xafenias et al ., 2015; Zhou et al ., 2013; Zhou et al ., 2015).
On the other hand, metabolically engineered fermentative bacterial strains are another feasible option, for instance, by adding the property of electroactivity (Moscoviz et al ., 2016). This approach has been confirmed by adopting the strains (e.g., c -type cytochromes CymA, MtrA, STC) from electroactive bacteria ( Shewanella oneidensis ) to fermentative bacteria ( Escherichia coli ), where the electron transfer process can be greatly improved (e.g., by 183%) (Sturm-Richter et al ., 2015). Alternatively, electroactive bacterial species (e.g., Shewanella oneidensis ) can also be engineered to utilize a variety range of substrates and organic wastes to further aid the whole EF processes (Flynn et al ., 2010).
1.3 Value-Added Products from Electro-Fermentation
To date, electro-fermentation has been investigated for a wide variety of value-added products, including carboxylates, alcohols, biopolymers, and other platform chemicals (see Table 1.1). This section reviews the studies related to EF for producing different value-added products.
Table 1.1 Summary of electro-fermentation and electro-selective fermentation for value-added bioproducts.
| Product |
Feedstock |
Inoculum |
System configuration |
Total working volume (L) |
Temperature (°C)/initial pH |
Applied voltage/potential |
Working electrode |
Reference |
| Butanol |
Glucose |
C. pasteurianum |
Dual chamber |
900 |
37/6.7 |
0-2.6 V |
Cathode |
(Mostafazadeh et al ., 2016) |
| Butanol |
Glucose |
Clostridium pasteurianum DSM 525 |
Dual chamber |
900 |
37/6.5 |
+0.045 V vs. SHE |
Cathode |
(Choi et al ., 2014) |
| Ethanol |
Glycerol |
Clostridium cellobioparum , + G. sulfurreducens |
Dual chamber |
190 |
30/6 |
0.24 V vs. Ag/AgCl |
Anode |
(Speers et al ., 2014) |
| Ethanol |
Glycerol |
Escherichia coli |
Dual chamber |
50 |
37/7.4 |
−44 mV vs. SCE |
Anode |
(Sturm-Richter et al ., 2015) |
| Ethanol |
Cellobiose |
G. sulfurreducens + Cellulomonas uda |
Single chamber |
1000 |
30/6.97 |
0.24 V vs. Ag/AgCl |
Anode |
(Awate et al ., 2017) |
| Ethanol |
Food waste |
Mixed culture |
Single chamber |
400 |
30/6.8 |
- |
- |
(Chandrasekhar et al ., 2015) Acetone-Butanol- |
| Ethanol (ABE) |
Glucose |
C. acetobutylicum |
Dual chamber |
240 |
37/6.8 |
-600 mV vs. Ag/AgCl |
Anode |
(Engel et al ., 2019) |
| 1,3-propanediol |
Glycerol |
Mixed-culture + G. sulfurreducens pre-colonized cathode. |
Dual chamber |
900 |
37/7 |
-900 mV vs. SCE |
Cathode |
(Moscoviz et al ., 2018) |
| 1,3-propanediol |
Glycerol |
Mixed culture |
Dual chamber |
520 |
21/6.9 |
−0.80 V to −1.10 V vs. SHE |
Cathode |
(Xafenias et al ., 2015) |
| 1,3-propanediol |
Glycerol |
Clostridium pasteurianum DSM 525 |
Dual chamber |
900 |
37/6.5 |
+0.045 V vs. SHE |
Cathode |
(Choi et al ., 2014) |
| Butyric acid |
Glucose |
Mixed culture |
Dual chamber |
540 |
25/5.5 |
-700 mV vs. SHE |
Cathode |
(Paiano et al ., 2019) |
| 3-hydroxypropionic acid |
Glycerol |
Recombinant Klebsiella pneumoniae L17 |
Dual chamber |
620 |
37/6 |
+0.5 V vs. Ag/AgCl |
Anode |
(Kim et al ., 2017) |
| Mixed VFAs |
Off gases from fermentation (CO 2+H 2) |
Mixed culture acclimatized homoacetogens |
Dual chamber |
400 |
37/6.5 |
-1.0 V vs. SHE |
Cathode |
(Zhou et al ., 2019) |
| Caproate |
Ethanol, acetate |
Mixed culture |
Dual chamber |
240 |
37/7.2 |
−0.8 V and −1.1 V vs. Ag/ AgCl |
Cathode |
(Jiang et al ., 2020) |
| Iso-butyrate |
Glucose, ethanol, and acetate |
Mixed culture |
Dual chamber |
540 |
-/5.6 |
-700 mV vs.SHE |
Cathode |
(Villano et al .,2017) |
| Lipid |
Microalgae biomass |
Scenedesmus acutus |
Dual chamber |
340 |
30/7.5 |
-0.3 V vs.Ag/AgCl |
Anode |
(Liu et al ., 2019) |
| Lipid |
Microalgae biomass |
Scenedesmus acutus |
Dual chamber |
200 |
25/7.0 |
-0.3 V vs.Ag/AgCl |
Anode |
(Liu et al ., 2020c) |
| Lipid |
Microalgae biomass |
Scenedesmus acutus |
Dual chamber |
200 |
-/- |
- |
Anode |
(Liu et al ., 2020b) |
| Acetoin |
Lactate |
Shewanella oneidensis |
- |
270 |
-/- |
0 mV vs. NHE |
Anode |
(Bursac et al ., 2017) |
| Acetoin |
Glucose |
Escherichia coli |
Dual chamber |
23 |
/-/ |
0.2 mV vs. NHE |
- |
(Förster et al ., 2017) |
| Polyhydroxybutyrate (PHB) |
Glycerol |
Ralstonia eutropha H16 |
Single chamber |
500 |
-/- |
10 mA |
Anode |
(Lai & Lan, 2020) |
| L-lysine |
Glucose |
Corynebacterium glutamicum |
Dual chamber |
360 |
20/7.0 |
-1.25 V |
Cathode |
(Xafenias et al ., 2017) |
| L-lysine |
Glucose |
Corynebacterium glutamicum |
Dual chamber |
350 |
30/7.2 |
0.697 V vs. SHE |
Anode |
(Vassilev et al ., 2018) |
Читать дальше














