Table 1.1 Cells of the innate and adaptive immune system discussed in this chapter.
|
Cell type |
Functions |
Location |
Cytokines produced |
Expressed molecules |
| Innate |
Keratinocyte |
Mechanical barrier, epidermal production Immune function |
Epidermis |
IL‐1, IL‐6, TNF, IL‐8, IL‐10, IL‐12, IL‐15, IL‐18, IL‐19, IL‐20, TGF, IL‐20, IL‐23, GM‐CSF, G‐CSF |
TLR, MHC‐I, MHC‐II, AMP |
| Langerhans cell |
Antigen‐presenting cell |
Epidermis |
IL‐12, IL‐23, IL‐6, TNF |
Fc and mannose receptors, ICAM‐1, IL‐12, MHC‐II |
| Dermal dendritic cell |
Antigen‐presenting cell |
Dermis |
IL‐12, IL‐23, IL‐6, TNF |
Fc and mannose receptors, ICAM‐1, IL‐12, MHC‐II |
| Mast cell |
Hypersensitivity reactions, vasodilation, chemotaxis, inflammation |
Dermis |
TNF, IL‐1, IL‐4, IL‐5, IL‐6, IL‐13, CCL3, CCL4, IL‐3, GM‐CSF |
TLRs, NF‐kB, NFAT, AP‐1 |
| Eosinophil |
Hypersensitivity reactions, vasodilation, chemotaxis, inflammation |
Dermis |
IL‐3, IL‐5, IL‐8, IL‐10, leukotrienes, GM‐CSF, hydrolases |
Fc receptor |
| Neutrophil |
Innate immunity, phagocytosis |
Dermis |
ROS, proteolytic enzymes |
TLRs, lectin receptor, mannose receptor |
| M1 macrophage |
Phagocytosis, antigen presentation, bactericidal activity |
Dermis |
IL‐6, IL‐12, TNF, iNOS |
JAK1, JAK2, STAT1, STAT2 |
| M2 macrophage |
Phagocytosis, antigen presentation, regenerative effects |
Dermis |
IL‐10, TGF, arginase‐1 |
JAK1, JAK2, JAK3, STAT6 |
| ILC (Innate lymphoid cell) |
Innate immunity |
Dermis |
IL‐1, IL‐23, IL‐25, IL‐33, TSLP |
Id2, T‐bet, GATA 3, ROR |
| Both innate and adaptive |
γ/δ T cell |
Elimination of intracellular microorganisms and infected cells; cell death |
Dermis |
IL‐17, IFN |
MHC‐ I |
| Natural killer cell |
Innate immunity against viruses and intracellular bacteria |
Dermis |
IFN, GM‐CSF, IL‐3 |
MHC‐I |
| Natural killer T cell |
Elimination of lipid antigens |
Dermis |
IL‐4, IL‐17, IL‐22, IFN |
MHC‐1 |
| Adaptive |
B lymphocyte |
Humoral response |
Dermis |
IL‐2, IL‐4, IL‐6, IL‐11, IL‐13, TNF, BAFF |
Antibodies |
| T‐helper lymphocyte |
Coordinates immune function |
Dermis |
Various depending on the type of T‐helper cell |
Various depending on the type of T‐helper cell |
| T‐cytotoxic lymphocyte |
Elimination of intracellular microorganisms and infected cells |
Dermis |
IFN |
Perforin, granzyme, granulysin |
| T‐regulatory lymphocytes |
Control of immune response |
Dermis |
IL‐10, TGF |
FoxP3, STAT5 |
Key: AMP, antimicrobial peptides; AP, activator protein; BAFF, B‐cell activating factor; CCL, chemokine ligand; FoxP3, forkhead box P3 protein; GATA, transcription factor; G‐CSF, granulocyte colony‐stimulating factor; GM‐CSF, granulocyte macrophage colony‐stimulating factor; ICAM, intercellular adhesion molecule; Id2, DNA binding protein inhibitor 2; IFN, interferon; IL, interleukin; iNOS, inducible nitric oxide synthase; JAK, Janus kinase; MHC, major histocompatibility complex; NFAT, nuclear factor of activated T cell; NF‐kB, nuclear factor kappa B; ROR, retinoic acid‐related orphan receptors; ROS, reactive oxygen species; STAT, signal transducer and activator of transcription; T‐bet, T box transcription factor; TGF, transforming growth factor; TLR, Toll‐like receptor; TNF, tumor necrosis factor; TSLP, thymic stromal lymphopoietin.
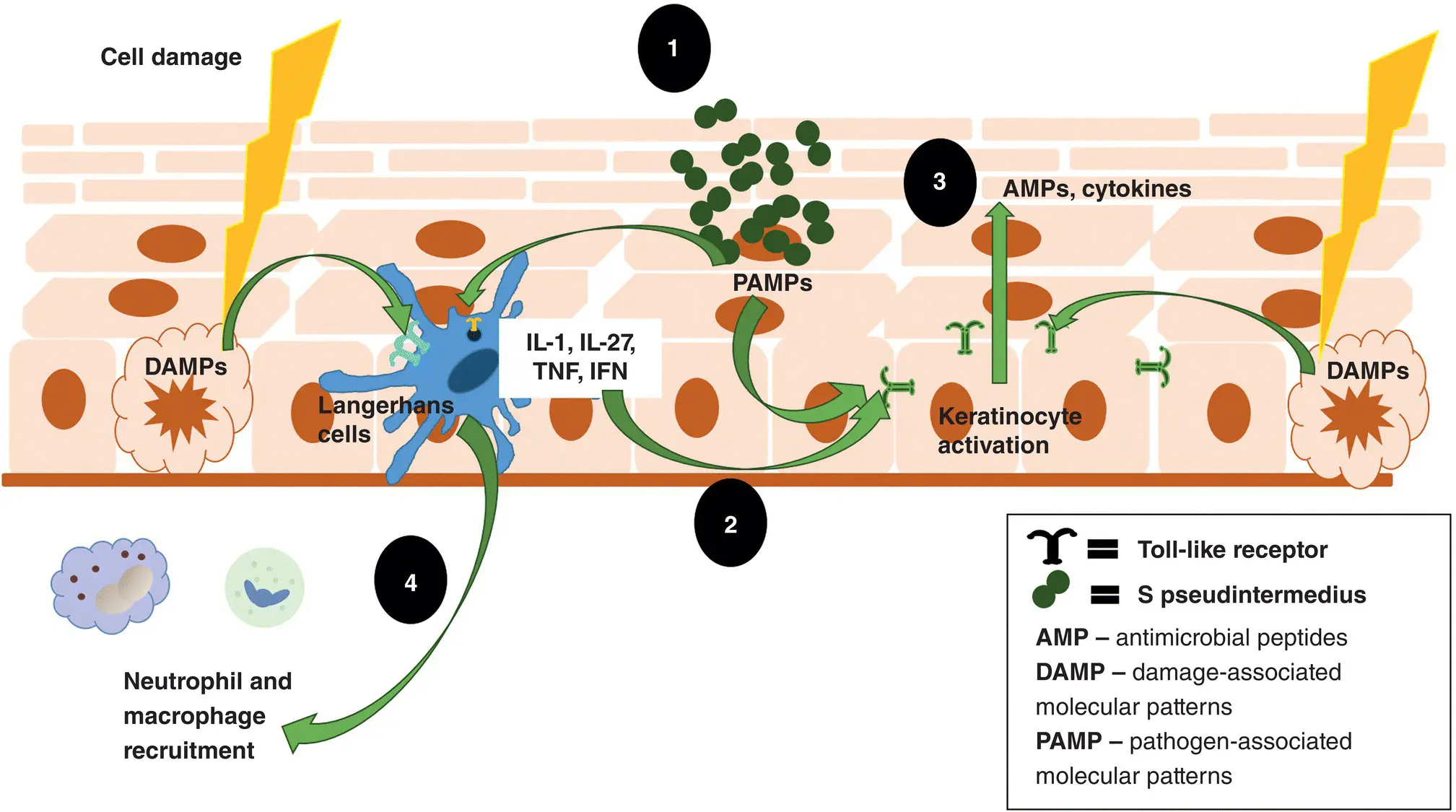
Figure 1.1 Innate skin immune system. 1. The innate immune system is activated when damage‐associated molecular pattern (DAMP) molecules released by damaged cells or pathogen‐associated molecular pattern (PAMP) molecules on pathogens are recognized by pattern‐recognition receptors such as Toll‐like receptors on both dendritic cells (Langerhans cells) and keratinocytes. 2. This leads to the activation of keratinocytes directly and the release of cytokines by the Langerhans cells that also activate the keratinocytes. 3. The activated keratinocyte releases various cytokines and AMPs to directly destroy the invading pathogen. 4. Neutrophils and macrophages are recruited to directly destroy the invaders. IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
Immune System of the Skin
The skin is a complex organ composed of an outermost layer, the epidermis; a middle layer, the dermis and cutaneous appendages; and an inner layer, the subcutis. Immune cells and inflammatory mediators are active in all these layers. It is important to understand that immune cells and inflammatory mediators are extremely interconnected and work in concert, as opposed to having isolated effects. If we compare the two branches of the immune system to an army, the innate immune system would be the entrenched peacekeeping force while the adaptive immune system would be the cavalry called in as reinforcements. By constantly communicating via cytokines and chemokines, they work together as one system to protect the host.
The major physical defense of the skin is the stratum corneum. This outermost layer continuously sheds into the environment, taking pathogens along with it. In addition to keratinocyte exfoliation, compounds present in the intercellular lipid cement create an environment unfavorable to pathogen invasion. These compounds include sodium chloride, albumin, complement components, transferrin, interferons, lipids, and antibodies donated by the adaptive immune system.
Below the stratum corneum the living epidermis is composed mainly of keratinocytes that are bound together by various junctional structures, including desmosomes, hemidesmosomes, and tight junctions. In addition to forming the major structural component of the epidermis, keratinocytes are many times the first to detect pathogens. By initiating the immune response, they act as sentinel cells for both the innate and adaptive immune systems. Keratinocytes produce small quantities of AMPs on a regular basis. AMPs are small cationic molecules that are a first line of defense against pathogens. They block lipopolysaccharides (a major negatively charged component of the outer membrane of gram‐negative bacteria), directly kill microbes, and induce histamine release from mast cells. Keratinocytes also detect highly conserved microbial surface structures (e.g. lipopolysaccharides, flagellins, teichoic acids) called pathogen‐associated molecular pattern (PAMP) molecules via pattern‐recognition receptors such as Toll‐like receptors. Other pattern‐recognition receptors on the keratinocyte surface detect damage‐associated molecular pattern molecules (DAMP) that are endogenous ligands/markers produced by injured or dying host tissue. These are important in the detection of irritants and toxins. Once pattern‐recognition receptors are triggered, the keratinocyte is activated. Depending on the type of assault and which pattern‐recognition receptors are triggered, the activated keratinocyte then produces more AMPs, and various pro‐inflammatory cytokines (a large group of small molecules important in cell signaling). Some of these cytokines have immediate effects on the invaders (innate response) that directly neutralize the pathogen, while others are important in recruiting and activating B and T lymphocytes (adaptive response). Once activated, keratinocytes express a wider variety of pattern‐recognition receptors. Keratinocytes can also act as antigen‐presenting cells for the adaptive immune response. They can express both major histocompatibility complex molecules (MHC) I and II on their surface. They are not able to prime naïve T cells, but can present antigen to both T‐helper (CD4+) and cytotoxic (CD8+) T lymphocytes.
Читать дальше