Mohamed N. Rahaman - Materials for Biomedical Engineering
Здесь есть возможность читать онлайн «Mohamed N. Rahaman - Materials for Biomedical Engineering» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Materials for Biomedical Engineering
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Materials for Biomedical Engineering: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Materials for Biomedical Engineering»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
A comprehensive yet accessible introductory textbook designed for one-semester courses in biomaterials Materials for Biomedical Engineering: Fundamentals and Applications
Materials for Biomedical Engineering: Fundamentals and Applications
Materials for Biomedical Engineering — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Materials for Biomedical Engineering», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
5.4.1 Surface Charging Mechanisms
The surface of most metals and ceramics used as biomaterials, normally hydroxylated due to chemically adsorbed water molecules, acquire a surface charge typically by adsorption of hydrogen (H +) ions, equivalent to hydronium (H 3O +) ions, or hydroxyl (OH −) ions ( Figure 5.13). These ions are often referred to as the charge‐determining ions for these materials. In an acidic medium (lower pH), preferential adsorption of H +ions leads to a positively charged surface, whereas in a basic medium (higher pH), a negatively charged surface is formed due to preferential adsorption of OH −ions. At some intermediate pH, called the point of zero charge (PZC), there is a balance between adsorption of H +and OH −ions, which leads to an electrically neutral surface. The PZC depends on the acidity or basicity of the surface composition. The more acidic oxides such as SiO 2have a lower PZC whereas the more basic oxides such as MgO have a higher PZC.
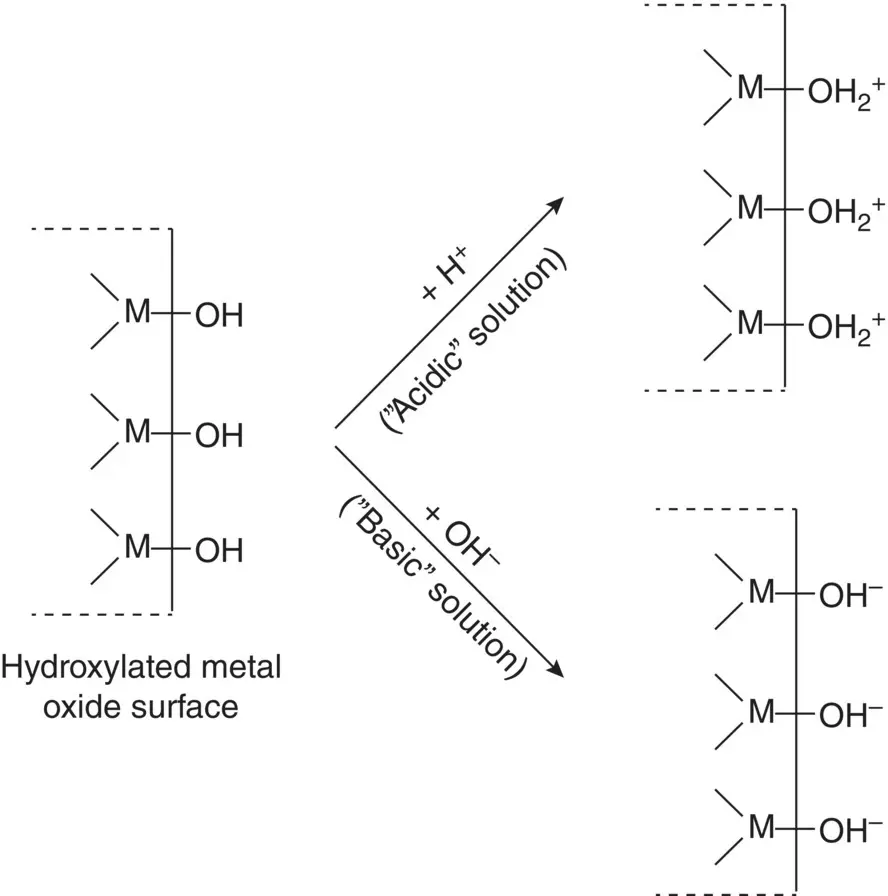
Figure 5.13 Production of surface charge on a hydroxylated metal oxide surface by adsorption of ions from an “acidic” or “basic” solution.
The PZC can be measured from acid–base titrations but, often, it is easier to measure the zeta ( ζ ) potential corresponding to the electrostatic potential at a small distance from the surface (a few tenths of a nanometer). The pH at which the measured ζ potential is zero is referred to as the isoelectric point (IEP). Upon implantation in the physiological environment, then, a material whose IEP is lower than ~7.4, such as a more acidic metal oxide, will have a negative surface charge and electrostatic potential, whereas one having an IEP higher than ~7.4, such as a more basic metal oxide, will have a positive surface charge and potential.
Some polymers that contain ionizable surface groups can acquire a charge by dissociation, such as dissociation of the H atom in the carboxyl (C=O)OH group, resulting in a negatively charged surface ( Figure 5.14). In comparison, the presence of amine (NH 2) groups can lead to a positively charged surface due to adsorption of H +ions from an aqueous medium. For a given composition, the extent of dissociation or adsorption and, thus, the magnitude of the surface charge depends on the pH of the aqueous medium. The dissociation of H +from the (C=O)OH group, for example, often starts at a pH of ~3 and is essentially completed at a pH of ~7 to 8 when a large fraction of the H atoms at the surface has dissociated. In comparison, NH 2groups are essentially neutral at pH higher than ~10 but the fraction at the surface that is protonated increases at lower pH.
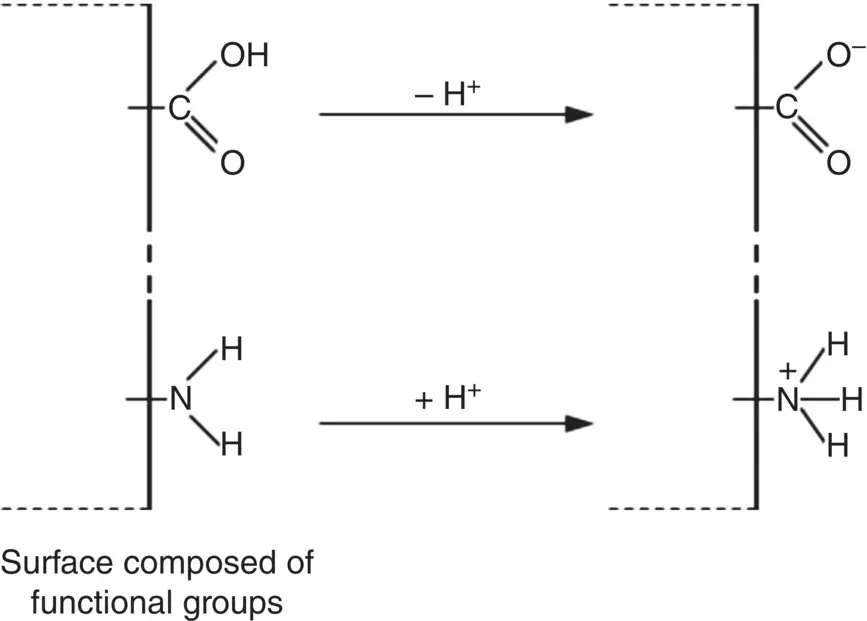
Figure 5.14 Production of negative or positive surface charge on surface composed of functional groups, as exemplified by the carboxyl and amine groups.
The presence of ionizable functional groups at the surface of a material is not a prerequisite for the acquisition of a surface charge. Polymers devoid of such functional groups can acquire a surface charge in aqueous media by dispersion forces, that is, van der Waals forces of attraction between the material and ions present in the aqueous medium. These attractive forces are similar in nature to the van der Waals forces between molecules described in Chapter 2. Ions in aqueous solutions are normally hydrated, that is, they are surrounded by an adsorbed layer of water molecules. Attraction between the atoms at the material's surface and the hydrated ions, positive or negative depending on the surface composition, leads to adsorption and, thus, to the production of a charged surface ( Figure 5.15). The aqueous medium of the physiological environment, for example, contains a variety of ions such as H +, Na +, K +, Mg 2+, Ca 2+, OH −, Cl −, HCO 3 −, HPO 4 2−, and SO 4 2−, and a variety of biomolecules ( Chapter 14). The ensuing zeta potential of the biomaterial reflects the nature of the ions or molecules adsorbed at its surface.
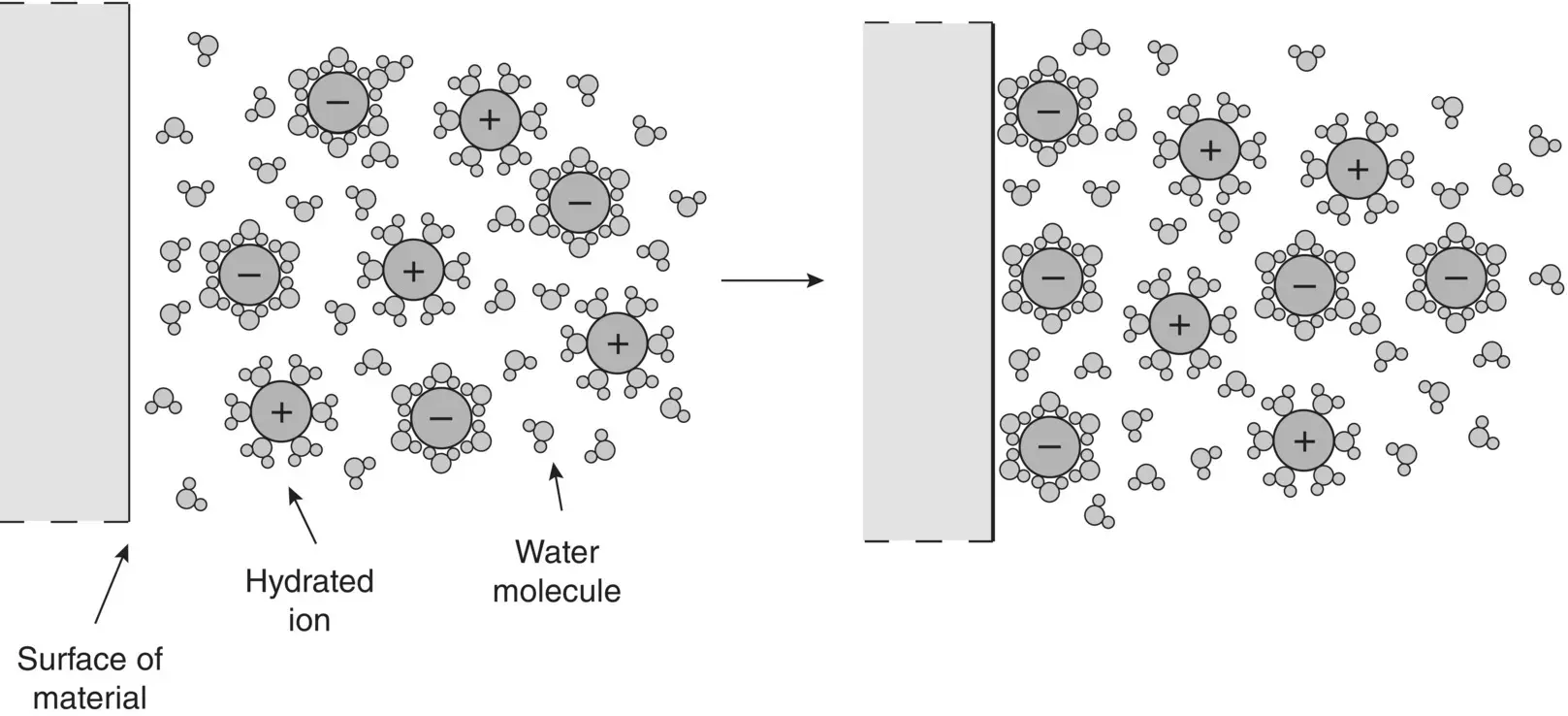
Figure 5.15 Production of surface charge on a surface devoid of functional groups by van der Waals attraction of ions in solution.
Overall, then, in an aqueous medium, the surface of metals and ceramics acquire an electrostatic charge by adsorption of H +or OH −ions ( Figure 5.13) whereas polymers composed of certain surface functional groups can also acquire a surface charge by, for example, dissociation or adsorption of H +ions ( Figure 5.14). Adsorption of water molecules and ions of the opposite charge, called counterions, leads to the formation of an ion atmosphere that is spread out in the solution. In colloid chemistry, this ion atmosphere is referred to as an electrical double layer ( Figure 5.16). It consists of a rather tightly bound layer of counterions, referred to as the Stern layer, and a more diffuse layer in which the positive and negative ions migrate freely. The electrostatic potential at the boundary between the Stern layer and the more diffuse layer approximates the zeta potential. As noted earlier, polymers that do not possess appropriate surface functional groups acquire a surface charge by adsorption of hydrated ions from the solution ( Figure 5.15).
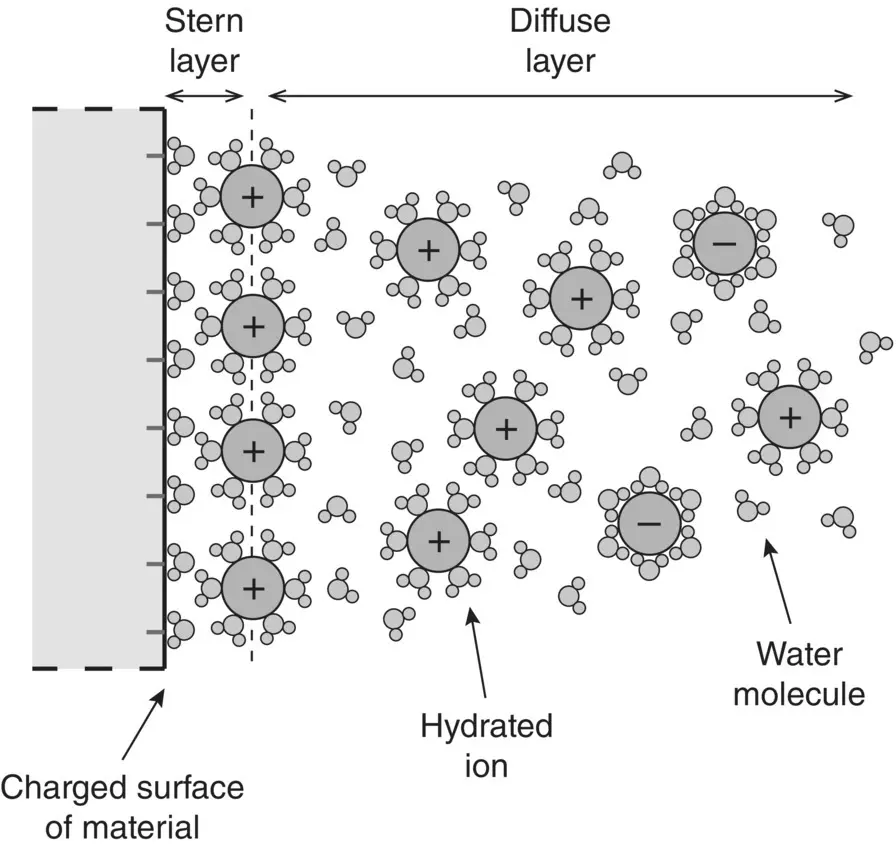
Figure 5.16 Illustration of the electrostatic charge distribution surrounding a solid surface upon introduction of the solid into an aqueous solution, shown for a solid that develops a negatively charged surface. The vertical dashed line corresponds approximately to the plane of the zeta potential.
5.4.2 Measurement of Surface Charge and Potential
Zeta potential measurements are often used to characterize the surface charge and surface potential of fine particles in a suspension using techniques that track the motion of the particles when an electric field is applied between two electrodes in the suspension. Although zeta potential measurements of macroscopic solids are more difficult to perform, they are more relevant to biomaterials used in their macroscopic solid form. This is because the zeta potential of a macroscopic solid is typically different from that of fine particles of the same nominal composition. The zeta potential of macroscopic solids is commonly determined from measurements of their streaming potential. Flow of an aqueous liquid over the charged surface of a material leads to shearing and shifting of the ions adsorbed at the surface. This leads to an electrokinetic effect called the streaming potential. The surface charge can be determined from the measured surface potential and the use of theoretical equations, but this is often not necessary because the zeta potential is commonly used as a measure of extent of the surface charge.
As an example, Figure 5.17shows streaming potential data for the zeta potential as a function of pH for three biomaterials, PEEK, a titanium alloy (Ti6Al4V), and silicon nitride (Si 3N 4), the same materials described in Figure 5.7(Bock et al. 2017). The pH of the aqueous liquid in these measurements was controlled using 0.1 M HCl solution at pH values in the range 3–5.5 and 0.1 M NaOH solution at pH between 5.5 and 10.0. In this medium, PEEK, Ti6Al4V, and Si 3N 4have an IEP of 3.9, 4.4, and 4.5, respectively, and, at a pH of 7.4 (equal to the homeostatic pH of the physiological medium), a negative zeta potential of −50 (extrapolated by nonlinear regression), −15, and −45 mV, respectively. As PEEK has no ionizable functional groups, its negative zeta potential at the homeostatic pH is presumably due to preferential adsorption of negative ions present in the medium, such as Cl −and OH −. The IEP of Ti6Al4V is in the measured range for TiO 2(~4–6) and, thus, the negative ζ potential at the homeostatic pH is most likely due to preferential adsorption of OH −ions at TiOH groups ( Figure 5.13). As the surface of Si 3N 4is composed of both, SiOH and NH 2groups, the IEP does not correspond to that (2–3) commonly observed for SiO 2. Instead, the higher IEP results from deprotonation and protonation reactions, respectively, at the SiOH and NH 2groups. Presumably, deprotonation at SiOH dominates at pH values above ~4.5, giving a negative surface charge and potential.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Materials for Biomedical Engineering»
Представляем Вашему вниманию похожие книги на «Materials for Biomedical Engineering» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Materials for Biomedical Engineering» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












