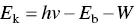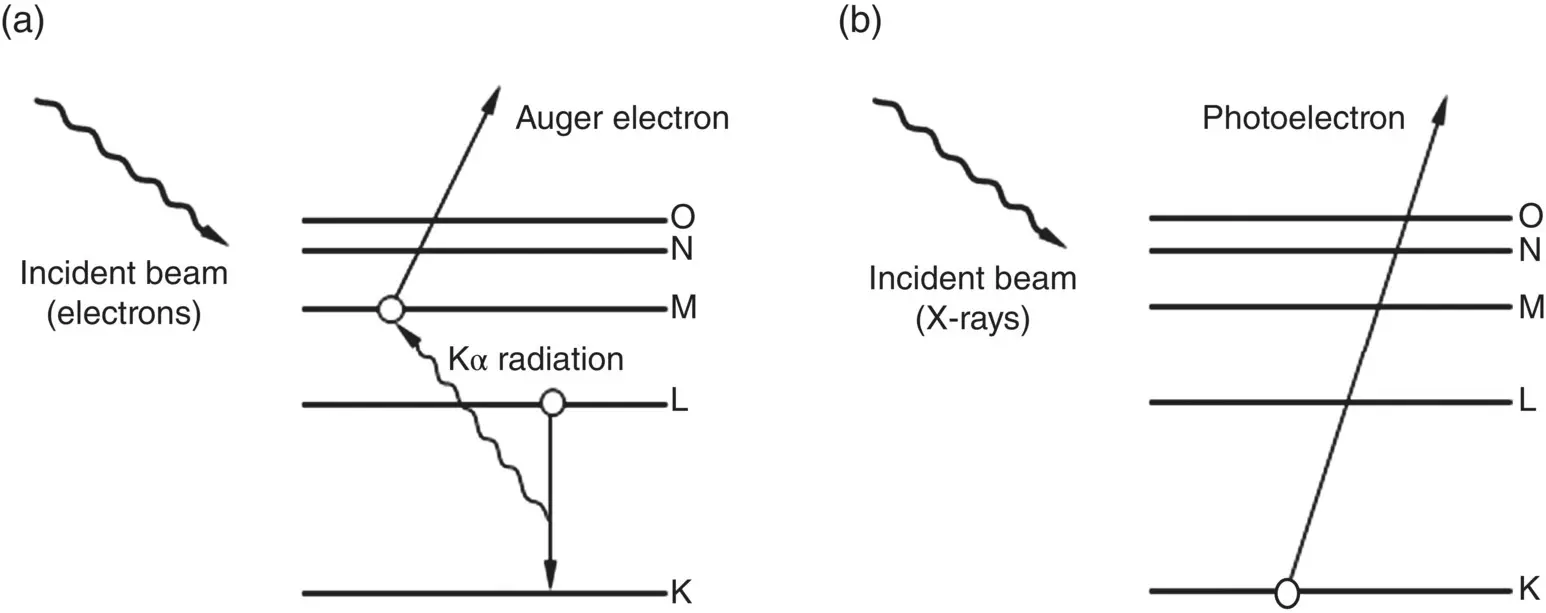
Figure 5.10 Interaction of incident beam (electrons or X‐rays) with a solid, producing atomic excitation with (a) emission of electrons followed by de‐excitation and emission of Auger electrons or (b) emission of photoelectrons.
X‐ray Photoelectron Spectroscopy (XPS)
In XPS, the specimen is irradiated with a low‐energy X‐ray beam that leads to the emission of electrons from the inner atomic orbitals by the well‐known Einstein photoelectric effect ( Figure 5.10b). The kinetic energy of the emitted photoelectrons E kis given by
(5.13) 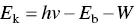
where h is the Planck constant, ν is the frequency of the incident X‐ray photons, E bis the binding energy of the photoelectron, and W is the work function of the spectrometer, a factor that corrects for the electrostatic environment in the instrument in which the photoelectron is produced and measured. By measuring E kin a spectrometer of known work function, E bcan be determined from Eq. (5.13). The data are plotted as the number of emitted electrons versus their binding energy. As the binding energy of an electron is characteristic of the atom and the orbital from which it is emitted, the spectrum can be used to provide information on the surface composition by comparing with standard spectra.
Because the photoelectrons typically have a higher energy than the Auger electrons, XPS is not as surface sensitive as AES, that is, AES can probe a shallower depth than XPS. However, by varying the emission angle at which the photoelectrons are collected, a technique known as angle‐resolved XPS, information from smaller and varying depths can be obtained. A benefit of XPS is that it can provide not just qualitative and quantitative information about the elemental surface composition but information about the chemical bonding (or oxidation state) of the surface atoms as well. As the incident beam is composed of X‐rays, XPS suffers from fewer problems related to electrostatic charging of the specimen and damage to the sample surface when compared to AES that relies on the use of an incident electron beam. Consequently, XPS is often a preferred technique for surface characterization of ceramics that are typically electrically insulating and polymers that are typically insulating and have a low hardness as well. However, as they provide similar information about elemental composition, XPS and AES tend to be used in a complementary manner.
A common mode of using XPS is to perform a survey scan over a wide binding energy range (typically 0–1000 eV) to provide a qualitative analysis of the surface composition. The fractional concentration of the elements present at the surface can be determined from the area of the major peaks of each element using software. Then, information about the chemical bonding or oxidation state of the relevant atoms can be determined from higher resolution scans of the relevant peaks and measuring their chemical shift, that is, the change in their binding energy. A variation in the number of valence electrons or the types of bonds that they form results in a change in the binding energy of the innermost electrons and, thus, to a chemical shift.
As an example, Figure 5.11shows an XPS survey spectrum of commercial purity titanium that, prior to analysis, was subjected to treatments commonly used for titanium implants, such as machining, cleaning with various solvents, and steam autoclaving (at 135 °C for 20 minutes) (Lausmaa 1996). The major peaks corresponding to Ti and O reveal an oxidized surface. The minor peak corresponding to C is often due to contamination and, in many cases, to carbon present adventitiously in the spectrometer. Except for Ti and O, the peaks corresponding to C and the minor elements were found to disappear or to decrease significantly after sputtering off a few nanometers of the surface, indicating that these elements were present mainly as impurities on the surface. A peak at ~459 eV, which, from reference spectra, corresponds to Ti in the compound TiO 2, dominates the high‐resolution spectrum of the Ti 2p peak ( Figure 5.12). A smaller peak at ~454 eV corresponds to Ti in the underlying metal. Overall, then, the surface oxide layer on this titanium specimen corresponds to TiO 2.
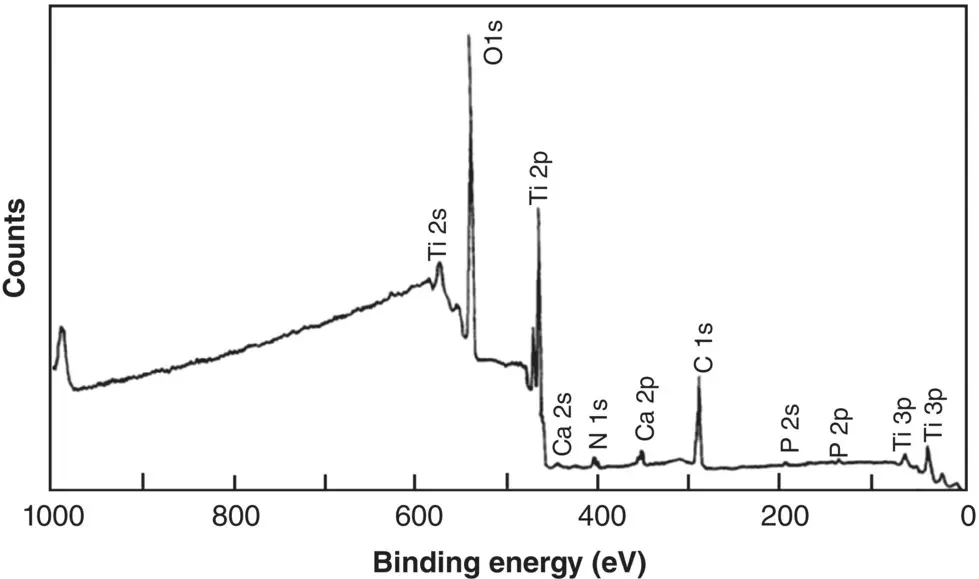
Figure 5.11 XPS survey spectrum for an autoclaved titanium dental implant.
Source: From Lausmaa (1996) / with permission of Elsevier.
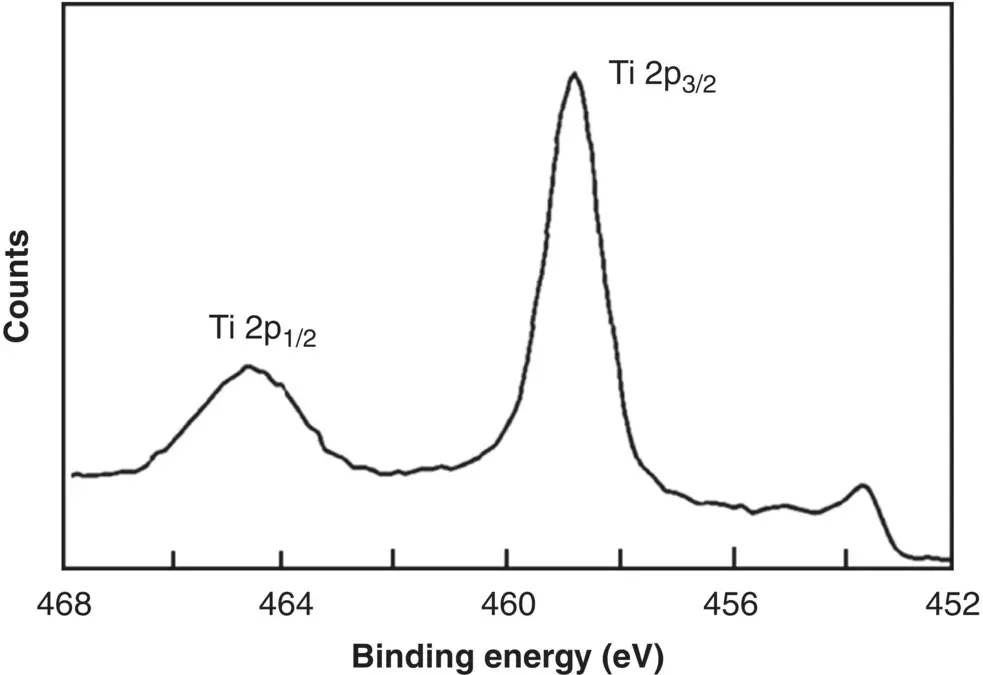
Figure 5.12 XPS high‐resolution spectrum of the Ti 2p peak for a machined titanium implant.
Source: From Lausmaa (1996) / with permission of Elsevier.
The alloy Ti6Al4V also sees considerable use as a biomaterial. When subjected to the same treatments, its XPS spectrum is similar to that of commercial purity titanium but, in addition, it often shows a small amount of Al 2O 3. Typically, the concentration of Al in the surface oxide layer is approximately the same as that in the interior of the alloy.
Secondary Ion Mass Spectroscopy (SIMS)
SIMS consists of bombarding a surface with a primary beam of Ar, Ne, or He ions and analyzing the emitted ions and ion clusters in a mass spectrometer. As the emitted ions and ion clusters are characteristic of the surface, SIMS provides information about the chemical composition of the surface. Some information on the chemical bonding of the atoms can also be extracted by analyzing the composition of emitted ions and ion clusters.
SIMS can be used in two distinct modes of analysis. In static SIMS, an ion beam of low intensity is used so that analysis is confined to the outermost layers of the surface. In comparison, in dynamic SIMS, a high intensity beam is used to erode successive atomic layers at a relatively rapid rate. Static SIMS is more relevant to the application of biomaterials as it provides information about the outermost surface atoms. While SIMS has the advantages of high spatial resolution, high sensitivity for qualitative elemental analysis and the ability to provide a detailed analysis of the chemical composition of the surface, quantitative analysis of the surface composition is often difficult.
As noted earlier, prior to implantation in the physiological environment, the surface of most polymers is normally covered with physically adsorbed water molecules. In comparison, most metals and ceramics have a surface composed of OH groups attached to the outermost metal atoms, on top of which are physically adsorbed H 2O molecules. The physiological fluid in vivo , on the other hand, can be approximated as an aqueous medium of homeostatic temperature 37.4 °C and pH 7.4, which contains a variety of ions, small molecules such as amino acids, macromolecules such as proteins, and substances released by cells. Upon implantation, the surface of a biomaterial acquires a positive or negative charge due to adsorption of ions or molecules from the aqueous medium or dissociation of certain surface functional groups, depending on the surface chemistry of the biomaterial.
Читать дальше