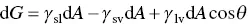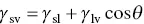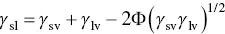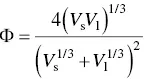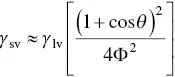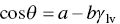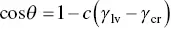Surface energy has a significant influence on reactions that take place at the surface of a material. As there is a thermodynamical driving force to lower its energy, a material with a high surface energy will tend to encourage adsorption of substances from its surroundings if this will lead to a reduction in energy. In this sense, the surface energy of a solid has often been discussed in terms of the degree of contact between a drop of liquid and the solid surface ( Figure 5.3). The change in the Gibbs free energy d G when the area A of the drop in contact with the solid increases by an infinitesimal amount d A is given by
(5.2) 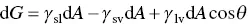
where γ sv, γ sl, and γ lvare the specific energies (energy per unit area) of the solid–vapor, solid–liquid, and liquid–vapor interfaces, respectively, and θ is the contact angle between the liquid and the solid (the angle between the tangent to the liquid–vapor interface at the contact point with the solid surface). At equilibrium, d G /d A = 0 and Eq. (5.2)gives
(5.3) 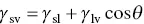
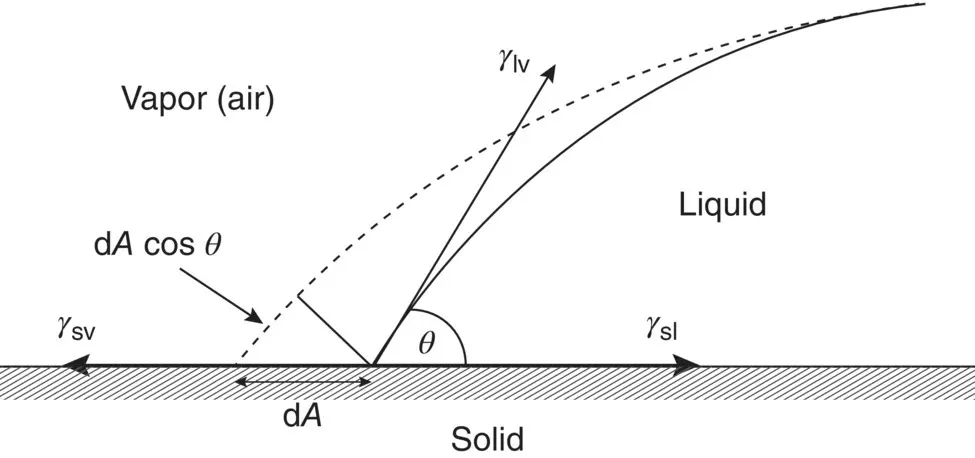
Figure 5.3 Contributions to the Gibbs free energy change due to change in area dA of a liquid drop on a solid.
Equation (5.3), sometimes referred to as the Young and Dupré equation, is what we would obtain by taking the horizontal components of the interfacial tensions in Figure 5.3. Roughly, good wetting of a solid by a liquid is said to be characterized by a low contact angle ( θ < 90°) and poor wetting by a high contact angle ( θ > 90°). Complete wetting and spreading of a liquid occurs when θ = 0° ( Figure 5.4).
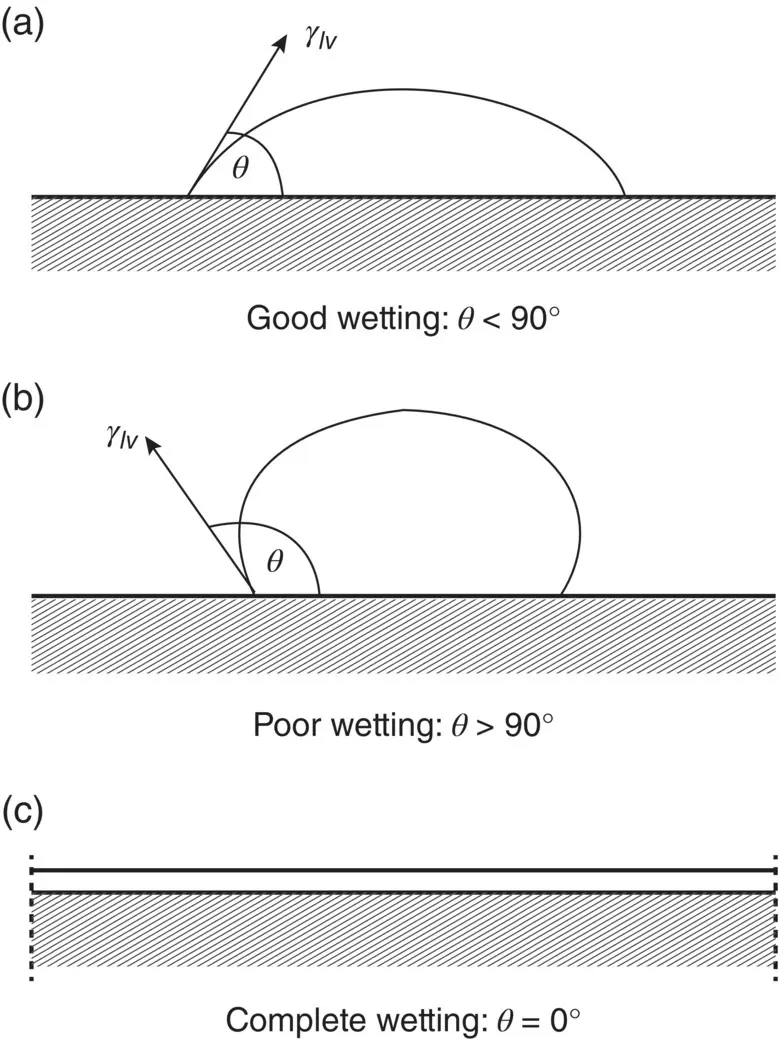
Figure 5.4 Wetting behavior between a liquid and a solid showing (a) good wetting, (b) poor wetting, and (c) complete wetting for a liquid of contact angle θ .
As the physiological fluid is aqueous in nature, the extent to which water will wet a biomaterial and spread over it has significant consequences for its interaction with the aqueous medium in vivo . A material that shows good wetting and spreading by water (low θ ) is referred to as hydrophilic (literally, water‐loving). If the solid has a higher surface energy than water, there is a thermodynamic driving force for wetting and spreading of the liquid in order to reduce the energy of the system. In comparison, a material that shows poor wetting by water (high θ ) is referred to as hydrophobic (literally, water‐hating). In this case, the material has a lower surface energy than water and, thus, wetting is thermodynamically unfavorable because it will lead to an increase in the energy of the system.
5.2.1 Determination of Surface Energy of Materials
According to Eq. (5.3), the surface energy γ svis related to the surface energy of the liquid γ lv, the solid–liquid interfacial energy γ sland the contact angle θ . Whereas γ lvand θ can be easily measured, γ svand γ slcannot. Consequently, methods for estimating γ svoften involve a combination of measuring γ lvand θ , and estimating γ slusing theoretical analyses, some of which can be fairly complex. While a variety of methods have been proposed, a useful method for polymers, based on its simplicity and accuracy, involves measuring θ for a single liquid of known surface energy (surface tension) γ lvand using a theoretical expression for γ sl(Girifalco and Good 1957):
(5.4) 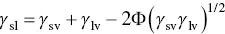
where Φ is expressed in terms of V sand V l, the molar volume (molecular weight divided by the density) of the molecules or molecular segments of the solid and the liquid, respectively, given by
(5.5) 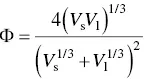
For a polymer, the repeating unit in the polymer chain can be taken to approximate the molecular segment. Using Eqs. (5.3)and (5.4), we get
(5.6) 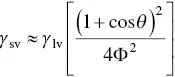
The measured contact angle of a water droplet on polymethyl methacrylate (PMMA) is 70°. Determine the surface energy of PMMA, given that the densities of PMMA and water are 1.2 and 1.0 g/cm 3, respectively, and the surface tension of water is 73.0 mN/m.
The molecular weight of water is 18.0 g/mol and the molecular weight of the repeating unit in PMMA is 100.1 g/mol, giving V l= 18.0 cm 3, and V s= 83.4 cm 3. Substituting in Eq. (5.5), we get Φ = 0.94. Then, substituting the values for Φ, γ lv, and θ in Eq. (5.6)gives γ sv= 37 mJ/m 2.
Another method applicable to materials of low surface energy, such as polymers, is referred to as the Zisman method. Fox and Zisman (1950, 1952) found that cos θ for a variety of polymers was approximately a monotonic function of γ lv, that is
(5.7) 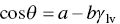
where a and b are constants. Eq. (5.7)can be expressed in the form
(5.8) 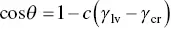
where c is a constant and γ cris a characteristic property of the material called the critical surface tension. The significance of γ cris that a liquid of surface tension less than or equal to γ crwill wet and spread over the material. This follows from the limiting condition for spreading is θ = 0° and the fact that the contact angle cannot be less than 0°. The Zisman method involves measuring the contact angle θ for several liquids of known surface tension on a given polymer and plotting cos θ versus γ lv( Figure 5.5). For many polymers, it is found that γ cris approximately equal to γ sv, as exemplified by the data for PMMA in Figure 5.5. However, because of the time‐consuming nature of the experiments and the less than rigorous theoretical justification, the Zisman method has found only limited application in recent years.
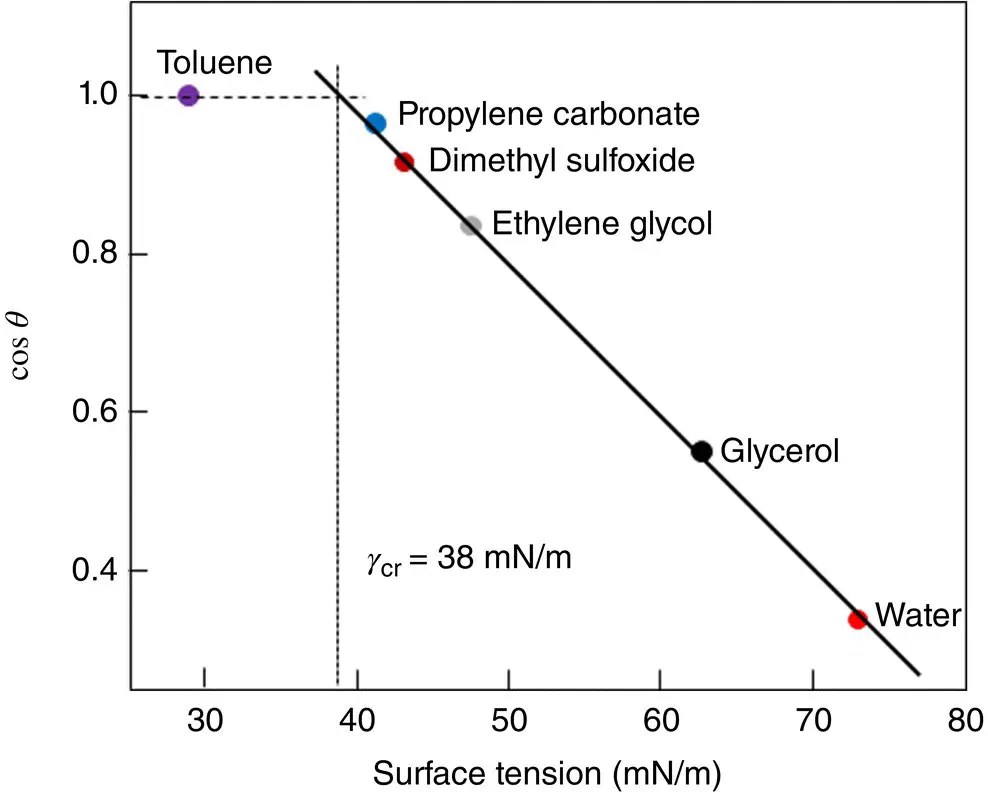
Figure 5.5 Zisman plot for polymethyl methacrylate (PMMA) using various liquids.
Читать дальше