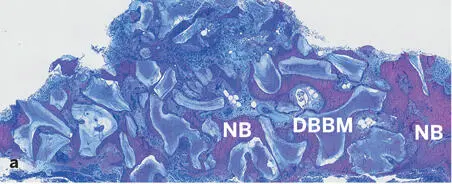
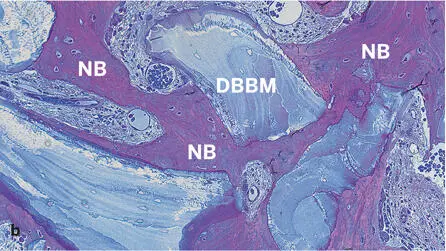
Fig 2-43Histologic section of a human biopsy specimen harvested 74 months after contour augmentation lateral to a dental implant with particulate autogenous bone, DBBM, and a collagen membrane (decalcified tissue; toluidine blue and basic fuchsin stain). Low (a) and high (b) magnifications demonstrate good tissue integration of DBBM particles. Newly formed bone (NB) covers a substantial portion of the DBBM surfaces and bridges neighboring DBBM particles. Adipocytes indicate presence of mature bone marrow.
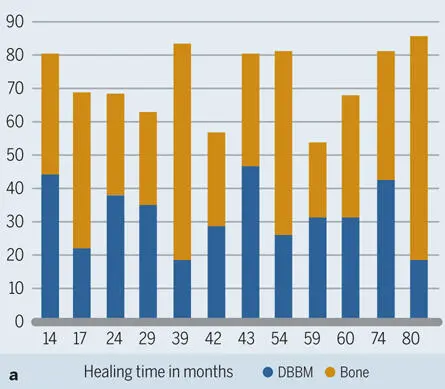
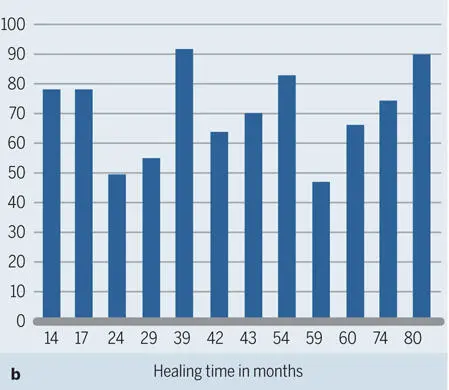
Fig 2-44Histograms for 12 human biopsy specimens harvested from 14 to 80 months after contour augmentation with particulate autologous bone, DBBM, and a collagen membrane. (a) Area fractions of new bone and DBBM. (b) Percentage of DBBM surface covered with new bone.
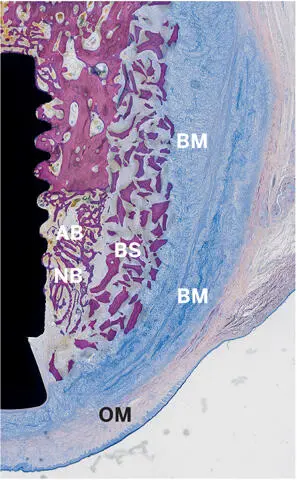
Fig 2-45Light micrograph demonstrating perfect structural integrity of an augmented site 3 weeks after contour augmentation with autogenous bone chips (AB), a bone substitute (BS) (Bio-Oss), and a non-cross-linked barrier membrane (BM) (Bio-Gide) of a buccal peri-implant bone defect in a dog mandible. Residues of the double-layered barrier membrane are still clearly visible, and the oral mucosa (OM) is blocked off. New bone (NB) has formed in the inner portion of the augmented region (undecalcified ground section; toluidine blue and basic fuchsin stain).
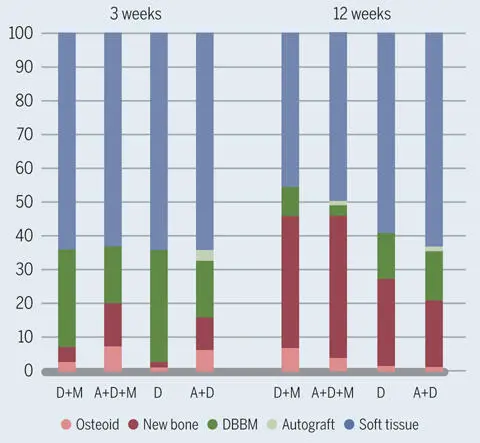
Fig 2-46Relative amounts of osteoid, new mineralized bone, DBBM, bone autograft, and soft tissue in the grafted region 3 and 12 weeks after contour augmentation in dog mandibles. Four combinations of 3 different materials were tested: (A) autogenous bone chips, (D) DBBM, and (M) collagen membrane. The addition of autogenous bone chips and the presence of the collagen membrane increased bone formation around DBBM particles.
Have we reached the limit with the contour augmentation technique using GBR? As we have seen, bone formation around bone substitutes lags behind bone formation around autogenous bone chips during early healing periods. Thus, it is tempting to find ways to improve the performance of bone substitutes. One possibility is to coat them with biologics like growth factors, a process called biofunctionalization . In particular, DBBM appears to be very suitable for this, since its high macro- and nanoporosity can absorb a lot of the patient’s own proteins and other macromolecules from the environment (Fig 2-47a) and can be precoated with biologically active molecules 137 , 138 (Fig 2-47b). In chapter 3of this book, the scientific background and value of biofunctionalizing biomaterials with bone-conditioned medium (BCM)—ie, the patient’s own growth factors released from bone—is discussed in detail.
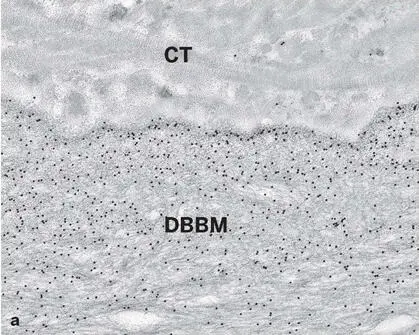
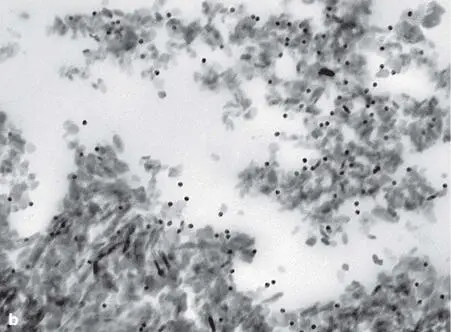
Fig 2-47 (a) Ultrathin section of a human biopsy specimen retrieved from a bony site augmented with DBBM and embedded in acrylic resin. High-resolution postembedding immunocytochemistry with an antibody against a typical bone-related noncollagenous protein demonstrates gold particle (black dots) labeling preferentially at the periphery of the DBBM particle. This finding indicates that DBBM takes up the patient’s own proteins from the wound environment after implantation. (b) High-resolution postembedding immunocytochemistry with an antibody against enamel matrix proteins demonstrates the ability of DBBM to be precoated with biologically active molecules prior to implantation. CT, soft connective tissue.
Conclusion
Bone has the unique capability to rebuild its original structure and function in response to a trauma. The trick in GBR is to harness this great regenerative potential to enhance bone formation around dental implants or at sites destined for implant placement. Under stable mechanical conditions, bone is formed directly or primarily, provided that two essential conditions are present: an ample blood supply and a solid base for bone deposition. The solid base is provided by the bony margins of a defect. Bone healing around dental implants is special in the sense that the implant surface in the defect region is devoid of bone, and thus bone has to grow into the defect from the walls of the preexisting bone. While the function of the barrier membrane is to keep away unwanted, fast-growing tissues, the functions of bone fillers are manifold. Since one type of bone filler cannot fulfill all requirements, the best of two worlds should be combined: autogenous bone with its great osteoconductive, osteoinductive, and osteogenic potential, boosting bone formation in the early healing phase; and a low-substitution filler that keeps the gained bone volume stable in the long term.
References
1.Bianco P. Structure and mineralization of bone. In: Bonucci E (ed). Calcification in Biological Systems. Boca Raton: CRC Press, 1992:243–268.
2.Weiner S, Traub W, Wagner HD. Lamellar bone: Structure-function relations. J Struct Biol 1999;126:241–255.
3.Frost HM. Micropetrosis. J Bone Joint Surg Am 1960;42-A:144–150.
4.Parfitt AM. The coupling of bone formation to bone resorption: A critical analysis of the concept and of its relevance to the pathogenesis of osteoporosis. Metab Bone Dis Relat Res 1982;4:1–6.
5.Miron RJ, Bosshardt DD. OsteoMacs: Key players around bone biomaterials. Biomaterials 2016;82:1–19.
6.Candeliere GA, Liu F, Aubin JE. Individual osteoblasts in the developing calvaria express different gene repertoires. Bone 2001;28:351–361.
7.Aubin JE, Triffit J. Mesenchymal stem cells and the osteoblast lineage. In: Bilezikian JP, Raisz LG, Rodan GA (eds). Principles of Bone Biology, vol 1, ed 2. San Diego: Academic Press, 2002:59–81.
8.Yang X, Karsenty G. Transcription factors in bone: Developmental and pathological aspects. Trends Mol Med 2002;8:340–345.
9.Otto F, Thornell AP, Crompton T, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 1997;89:765–771.
10.Bronckers AL, Engelse MA, Cavender A, Gaikwad J, D’Souza RN. Cell-specific patterns of Cbfa1 mRNA and protein expression in postnatal murine dental tissues. Mech Dev 2001;101:255–258.
11.Marks SC, Jr, Popoff SN. Bone cell biology: The regulation of development, structure, and function in the skeleton. Am J Anat 1988;183:1–44.
Читать дальше




















