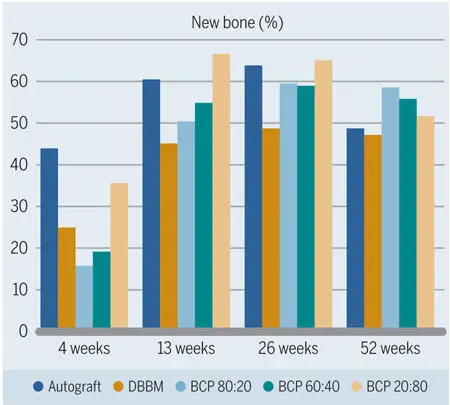
Fig 2-37Percentage of bone formation in standardized bone defects in the mandibles of minipigs after grafting. In the early healing phases, more new bone formation is seen in defects grafted with BCPs with high TCP content. (Data from Jensen et al. 120 )
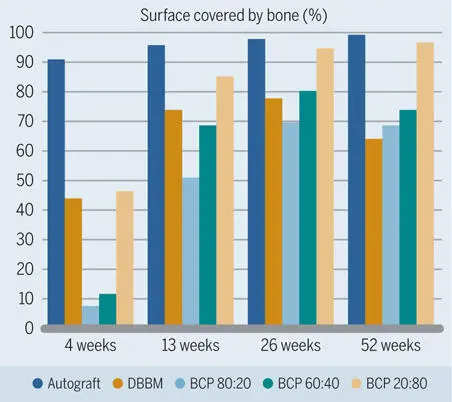
Fig 2-38Percentage of grafting material surface covered with new bone in standardized bone defects in the mandibles of minipigs. (Data from Jensen et al. 120 )
What has not been mentioned thus far is the HA of bovine origin. Various brands of bovine-derived bone substitutes are available on the market. However, one of them outclasses all other forms in terms of quantity of scientific data: Bio-Oss (Geistlich), also known as deproteinized bovine bone mineral (DBBM), bovine-derived bone mineral (BDBM), or anorganic bovine bone (ABB). Bio-Oss has been commercially available for regenerative dentistry for more than 30 years.
Some controversy remains as to whether DBBM is truly resorbable. 127 It has been shown in vitro that osteoclast progenitor cells are able to proliferate on DBBM surfaces, and later, as osteoclast-like cells, produce resorption pits. Compared with native bovine bone, however, the osteoclasts are reduced in number and size, and the resorption pits are less pronounced. 115 Experimental in vivo studies have also demonstrated multinucleated giant cells on DBBM surfaces that stain positive for TRAP 119 , 120 , 128 – 130 and occasionally display a sealing zone, a ruffled border, and shallow Howship lacunae 130 (Fig 2-39). These findings suggest that these cells have osteoclast-like properties, but fail to resorb this biomaterial, at least at a fast pace. Also, in the mandibular defect model in the minipig, a significant reduction in volume over observation periods of up to 1 year was not seen. 120 Human biopsies after sinus augmentation confirm that particles of DBBM can still be found up to 20 years postoperatively 131 (Fig 2-40). Thus, in daily practice, this particular xenograft must be considered very resistant to resorption.
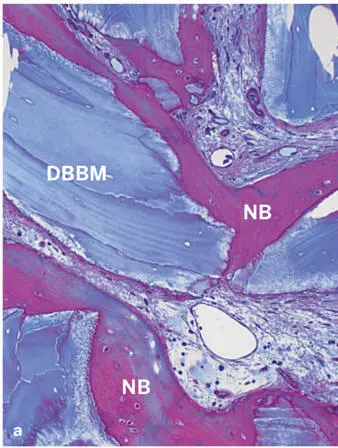

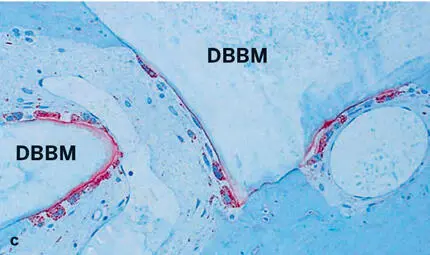
Fig 2-39Histologic sections of human biopsy specimens grafted with DBBM (thin sections of decalcified tissue). (a) The DBBM particles show good tissue integration. Newly formed bone (NB) covers part of the bone substitute surface and bridges neighboring DBBM particles (basic fuchsin and toluidine blue stain). (b) Osteoclast-like, multinucleated giant cells (arrows) are frequently seen lining the DBBM surface, which occasionally shows shallow resorption depressions (arrowheads) (toluidine blue stain alone). (c) Staining for tartrate-resistant acid phosphatase (TRAP) (red staining) identifies osteoclast-like, multinucleated giant cells on the surface of the DBBM particles (stained for TRAP and counterstained with toluidine blue).
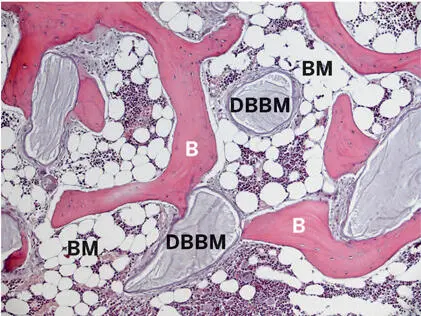
Fig 2-40Histologic section of a human biopsy specimen harvested 20 years after sinus floor elevation with DBBM. DBBM particles are interconnected by bone matrix (B) and surrounded by mature fatty bone marrow (BM) (thin paraffin section of decalcified tissue; toluidine blue and basic fuchsin stain).
In the studies by Jensen et al 120 , 125 and Broggini et al, 126 the amount of new bone in defects filled with DBBM increased over time, yet at a slower pace than around the autograft particles (see Figs 2-35 and 2-37). Likewise, osteoconductivity was lower with DBBM than with autograft particles (see Fig 2-38). This difference was clearly visible for up to 4 weeks after defect fill, and most pronounced after 1 to 2 weeks (Fig 2-41). Although bone regeneration started from the defect margins for all types of bone fillers, progression of bone formation and consequently defect fill with new bone was fastest when autogenous bone chips were used (Fig 2-42).
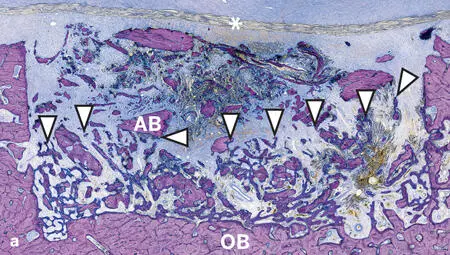
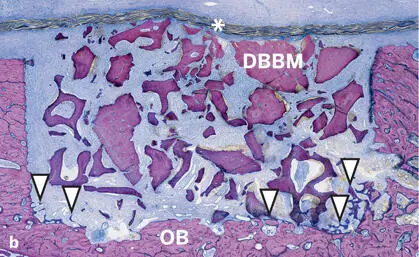
Fig 2-41Histologic sections of standardized bone defects in the minipig mandible 2 weeks after GBR with an ePTFE membrane (asterisks) and autogenous bone chips (AB) (a) or DBBM (b) (undecalcified ground sections; toluidine blue and basic fuchsin stain). The defect margins are delineated by old bone (OB) and the membrane. Formation of new bone (arrowheads) begins from the defect margins. New bone formation is much more advanced (arrowheads) in defects filled with autogenous bone than with DBBM.
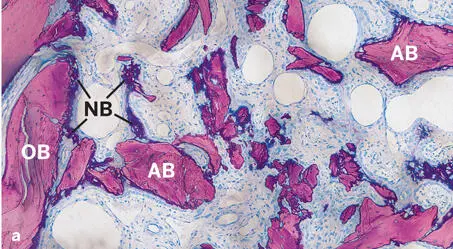
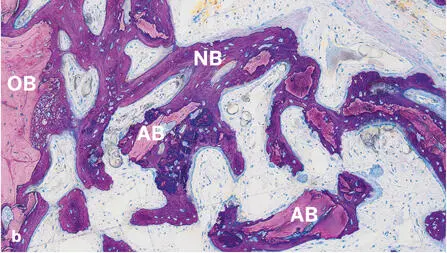
Fig 2-42Histologic sections of standardized bone defects in the minipig mandible 1 and 2 weeks after GBR with an ePTFE membrane and autogenous bone chips (AB) (undecalcified ground sections; toluidine blue and basic fuchsin stain). Formation of new bone (NB) begins from the surface of old bone (OB) at the defect margins and progresses towards the center of the defect. New bone formation is already visible after 1 week (a) and much more advanced after 2 weeks (b) .
These and other studies came to the conclusion that it would be advantageous to combine the best of two worlds: autogenous bone, with its excellent osteopromotive and osteoconductive properties, boosting bone formation in the early healing phase; and DBBM, with its low degradation rate, helping to keep the gained bone volume stable over the long term. Indeed, clinical and radiographic data from humans 132 – 135 have confirmed this biology-inspired concept, in which autogenous bone chips and DBBM are placed layer by layer to augment peri-implant bone defects. In this two-layer approach, the autograft particles are placed close to the implant surface to promote bone outgrowth from preexisting bone, and the DBBM particles form an external shield against resorption. Tiny biopsies harvested 14 to 80 months after contour augmentation in patients supplied histologic evidence of long-term stability when applying this concept. 129 While the histology has shown good integration of DBBM in newly formed bone (Fig 2-43), histomorphometry has demonstrated stable new bone volume and no signs of significant substitution of DBBM particles over time (Fig 2-44). Thus, earlier findings from preclinical studies were confirmed, and it was suggested that the low substitution rate of DBBM may be the reason for the clinically and radiographically documented long-term stability of contour augmentation, when a combination of autogenous bone chips, DBBM particles, and a collagen membrane is used. In a recent preclinical study, it was shown that the addition of autogenous bone chips and the presence of a collagen membrane increased bone formation around DBBM particles 136 (Figs 2-45 and 2-46).
Читать дальше






















