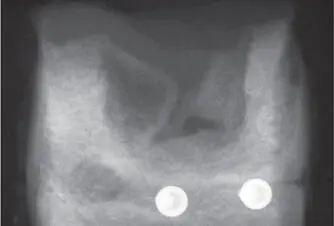
Fig 2-29Radiograph of a membrane-covered defect after 2 months shows bony ingrowth from the mesial and distal walls, as well as from the bottom of the defect. (Reproduced with permission from Schenk et al. 123 )
Formation of the primary spongeous scaffold.The histology of bone formation in the membrane-protected space exhibited a remarkable similarity to that found during bone development and growth (Fig 2-30). The infiltration of the hematoma by granulation tissue followed the basic pattern of wound healing. The invading vascular sprouts were accompanied by cells originating from the bone marrow at the periphery of the defect, thus enabling mesenchymal stem cells to differentiate into osteoblasts. 124 From the cut cortical and trabecular surfaces, woven bone sprouted out, mostly in the shape of thin, bifurcating plates (Fig 2-31). A particular characteristic of this primary cancellous scaffold is the perfect interdigitation with the vascular plexus. As already mentioned, angiogenesis and an ample blood supply are mandatory for bone development and maintenance.
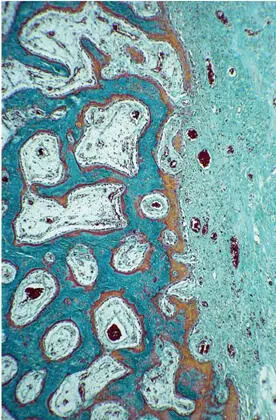
Fig 2-30Organization of the hematoma and woven bone formation. Blood vessels and bone-forming cells invade the former hematoma (right) and construct a scaffold of woven bone (Goldner trichrome stain).
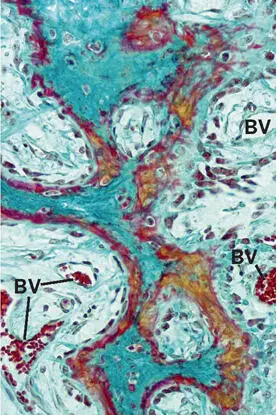
Fig 2-31In the advancing ossification front, blood vessels (BV) and outgrowing trabeculae are tightly interconnected. Goldner trichrome stain stains osteoid seams in red and mineralized bone matrix in green .
Transformation into compact bone and a regular spongiosa.While the original primary trabeculae consisted exclusively of woven bone, it later served as a template for the apposition of parallel-fibered bone followed by lamellar bone, which eventually would constitute both compact bone and a regular spongiosa with mature bone marrow. These events occurred 3 to 4 months after surgery (Fig 2-32).
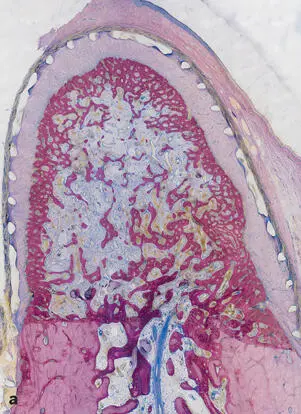
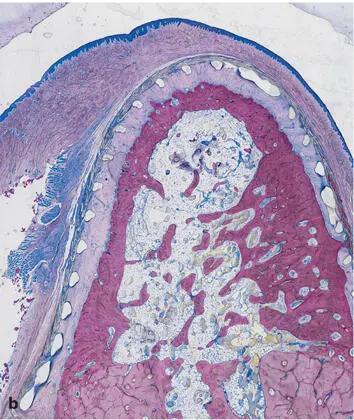
Fig 2-32Transformation of the primary spongiosa spongework into cortical bone and cancellous bone. (a) After 2 months, the spongiosa is denser at the periphery than in the center of the bony regenerate. (b) Cortical bone and secondary spongiosa after 4 months. A compact bone layer in the periphery confines a cancellous bone in the center with well-defined trabeculae and regular bone marrow (surface staining with toluidine blue and basic fuchsin.)
Remodeling of cortical bone.During the fourth month, the cortical bone entered its last phase of maturation: Haversian remodeling.
Modeling of the bony regenerate.At the end of the fourth month, growth and modeling of the bone within the membrane-confined space continued, particularly in the central portion. With the formation of a cortical bone layer, the periosteal and endosteal envelopes were also restored. As long as modeling was occurring, the bone surface was locally lined by osteoblasts and an osteoid seam, or was covered by osteoclasts or Howship lacunae, as a sign of ongoing or past resorptive activity.
Bone healing in membrane-protected defects with addition of a bone filler
The healing pattern of bone under a barrier membrane with the addition of various particulate bone fillers was analyzed in numerous experimental animal studies. One standardized animal model was introduced by Buser et al. 106 In this model, which excludes the interference of the particular conditions in the oral cavity, standardized bone defects were created in the mandibles of minipigs with extraoral access. This study showed that particulate bone fillers have different biologic characteristics with regard to both bone formation potential and bone filler degradation dynamics. After 4 weeks, which was the earliest observation period in this study, significantly more new bone was formed when autogenous bone was used as a filler, as compared to DFDBA, a synthetic β-TCP biomaterial, and coral-derived HA (Fig 2-33). 106 After 12 and 24 weeks, there was still more bone formation in the autogenous bone group than in the groups with DFDBA and coral-derived HA, but most new bone was found in the TCP group. On the other hand, TCP showed the greatest degradation rate, meaning that the volume of all three other fillers was more stable. Another important finding was that the autograft was the most osteoconductive filler material over the entire observation period (Fig 2-34). 106 From this study, it was concluded that defects filled with autograft clearly demonstrated the best results in the early phase of healing and that the TCP biomaterial used showed a fast degradation and substitution rate.
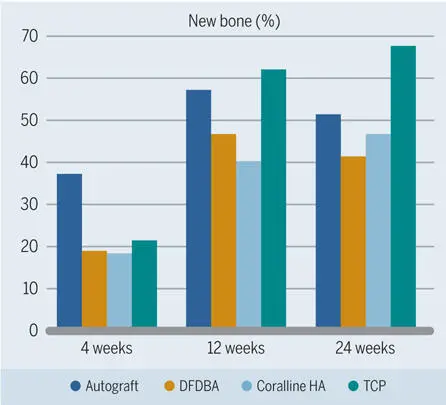
Fig 2-33Percentage of new bone in standardized bone defects in the mandibles of minipigs grafted with different materials. (Data from Buser et al. 106 )
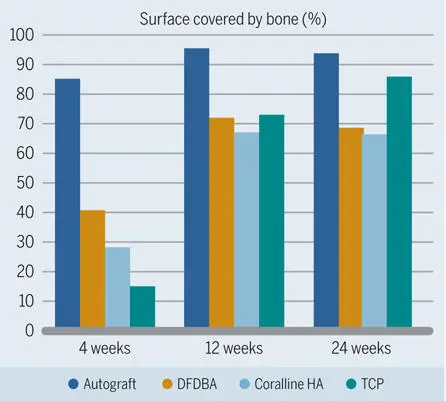
Fig 2-34Percentage of grafting material surface covered with bone as an indicator of the osteoconductive potential of different grafting materials in standardized bone defects in the mandibles of minipigs. (Data from Buser et al. 106 )
Based on these intriguing results, earlier observation periods down to 1 week were chosen, and other bone filler biomaterials were tested in subsequent studies. 119 , 120 , 125 , 126 Collectively, these studies confirmed that the autograft caused most new bone formation—at least up to 4 weeks (Figs 2-35 to 2-37)—and was the most osteoconductive filler material (Fig 2-38). It was also confirmed that the β-TCP biomaterial lagged behind but was catching up with the autograft group. Furthermore, these studies demonstrated that use of synthetic β-TCP led to faster bone formation than use of synthetic HA, and that biphasic BCP with a 60:40 ratio of HA and TCP was somewhere in between (see Fig 2-36). Varying the ratio of HA and TCP showed that the higher the TCP content was, the more quickly bone formation occurred (see Fig 2-37).
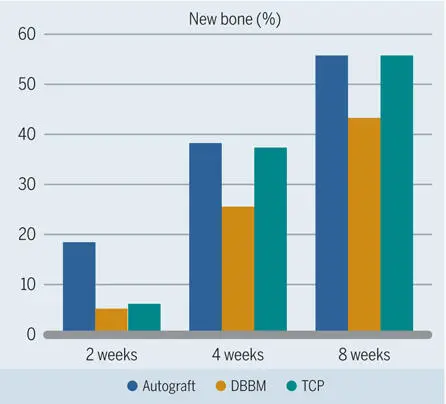
Fig 2-35Percentage of new bone in standardized bone defects in the mandibles of minipigs after grafting with different materials. (Data from Jensen et al. 125 )
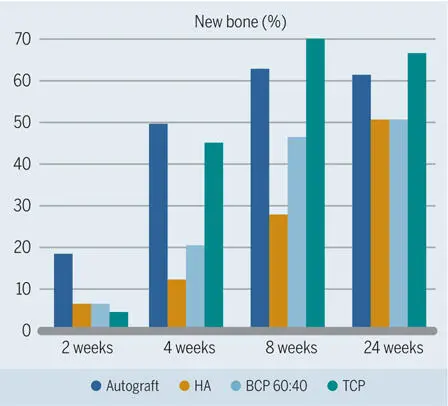
Fig 2-36Percentage new bone in standardized bone defects in the mandibles of minipigs after grafting with different materials. The three bone substitute materials have identical material characteristics except for the chemical composition. In the early healing phases, more new bone formation is seen in defects grafted with TCP than with BCP, which resulted in more new bone formation than HA. (Data from Jensen et al. 119 )
Читать дальше





















