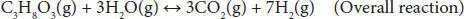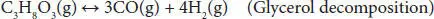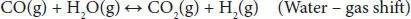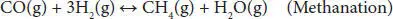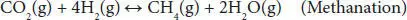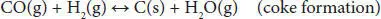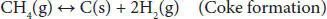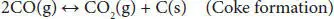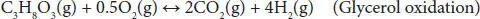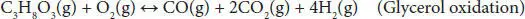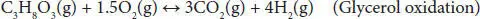4.4.2.3 Reforming of Glycerol
Hydrogen has been recognized as “the fuel of the future” because it generates only water as a product after combustion. In addition to its utilization as an energy source, hydrogen is consumed as raw material to obtain formic acid, hydrochloric acid, cyclohexane, and other important solvents and reagents. Moreover, it is consumed as a reactant in the hydrogenation process carried out in food industries and the hydrocracking process of petrochemical industries. Methane from fossil resources is used as raw material for approximately 95% of worldwide hydrogen production. Syngas is a mixture of carbon monoxide (CO) and hydrogen (H 2). It is a key reactant for several processes including ammonia synthesis, methanol production, Fischer–Tropsch synthesis, and H 2generation via fuel cells. Hydrogen/syngas obtained from renewable resources has the potential to be employed as an alternative within the contemporary fossil fuel-dependent energy landscape [8]. Given that global glycerol production continues to grow in the market, it can be used as an alternative for the large-scale production of hydrogen and syngas.
The thermochemical process is the most widely used route to generate the H 2/syngas. Among these, steam reforming of methane is a popular, fully developed, and efficient technology to produce H 2/syngas in industries [41]. Steam reforming has also been extensively explored for H 2generation from other hydrocarbons and alcohols (such as methanol and ethanol) [42]. Several reforming technologies, characterized as thermochemical routes, have been studied for hydrogen or syngas production from glycerol. The key technologies are steam reforming (SR), partial oxidation reforming (POR), autothermal reforming (ATR), and aqueous phase reforming (APR). Several important reactions associated with different reforming processes are as follow:
(4.1) 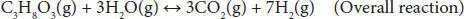
(4.2) 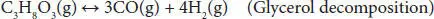
(4.3) 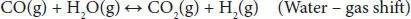
(4.4) 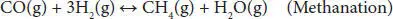
(4.5) 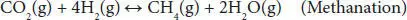
(4.6) 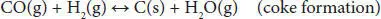
(4.7) 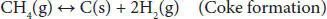
(4.8) 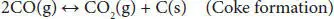
(4.9) 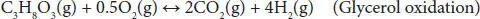
(4.10) 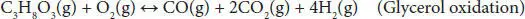
(4.11) 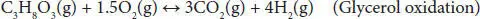
(4.12) 
The gaseous product of the glycerol reforming process contains constituents such as CO, H 2, and CO 2in major proportions. Gaseous products from these reforming processes also contain small fractions of compounds such as methane, ethylene, methanol, ethanol, acetic acid, and acetaldehyde. All of the glycerol reforming processes are generally performed in a fixed-bed reactor. Reforming processes are usually conducted with the help of a catalyst within the temperature range of 300–900 °C. The catalyst brings down the activation energy of the reforming process and favors the fast kinetic. To improve the suitability of the reforming processes for large-scale production, the catalyst must be highly active and stable, provides a high resistance for coke formation, exhibits the resistance for metal sintering, and suppresses undesirable side reactions such as methanation [41].
Steam reforming of glycerol has been identified as one of the most promising processes because the existing industrial plants of steam reforming of methane can be used for hydrogen or syngas production after slight modification. This process involves the glycerol reaction with steam on the surface of a catalyst and results in the generation of CO and H 2. The overall reaction for glycerol steam reforming can be represented by stoichiometric Equation (4.1), where the molar ratio of hydrogen to glycerol (7/1) is quite interesting. The overall reaction may be viewed primarily as the combination of Equation (4.2)(glycerol decomposition) and Equation (4.3)(water-gas-shift reaction) [9, 12]. Water-gas shift reaction results in the generation of additional H 2. The glycerol decomposition reaction is extremely endothermic, while the water-gas shift reaction is slightly exothermic, but the overall steam reforming is endothermic. Several studies have shown that this process shows the best result within the temperature range of 500–750 °C. Higher temperature increases the operating cost, energy requirement, and reactor cost. Lower temperature leads to a decrease in H 2selectivity due to the formation of CO 2and CH 4in higher amounts. The operating pressure generally used in this process is atmospheric. Lower pressure in the vacuum range facilitates the lower energy requirement, low reaction temperature, and reduce metal sintering in the catalyst.
The molar ratios of glycerol to water are commonly varied between 1/6 and 1/12. This molar ratio strongly affects the yield and selectivity of H 2. According to Le Chatelier’s principle, at the higher water/glycerol molar ratio, the equilibrium shift in the direction of more water utilization, and consequently more H 2is produced. A high water/glycerol molar ratio also promotes the gasification of carbon and suppresses its accumulation as coke over the catalytic active sites. However, the operating cost becomes high due to the requirement of excessive energy to vaporize the reaction mixture of high water/glycerol molar ratio.
In addition, other thermodynamic feasible reactions include methane formation ( Equations (4.4), (4.5)). The methanation reaction is favored at low temperatures and slightly influenced by pressure change. Coke formation also occurs in the steam reforming of glycerol ( Equations (4.6)– (4.8)). The coke accumulates over the surface of the catalyst, blocks the active sites, and eventually deactivates them. This coke is mainly produced by Boudouard ( Equation (4.7)) and methane cracking ( Equation (4.8)) side reactions [41, 42].
In partial oxidation, the amount of oxygen is supplied below the stoichiometric requirement of complete combustion. The overall POR reaction is commonly expressed by Equation (4.11). Since POR is an exothermic process, the amount of oxygen directly controls the energy efficiency of this process. The complete oxidation of glycerol can be represented using Equation (4.12). POR process occurs under atmospheric pressure. POR is an energy-efficient process and enables the generation of syngas through varying oxygen amounts. The coke formation is very low under an oxidative environment.
Читать дальше