Table 4.3 Performance of different carbon catalysts for different valorization processes.
| S. N. |
Name of catalyst |
Source of catalyst |
Type of process |
Reactant |
Glycerol derivatives |
Conversion of glycerol (%) |
Selectivity (%) |
Ref. |
| 1. |
TC-L carbon |
Rice husk |
Esterification |
Acetic acid |
DAG + TAG |
90 |
90 |
[37] |
| 2. |
TC-L carbon |
Rice husk |
Etherification |
TBA |
DTBG + TTBG |
53 |
25 |
[37] |
| 3. |
TAC-673 |
Sucrose |
Esterification |
Acetic acid |
TAG |
<99 |
50 |
[27] |
| 4. |
AC-SA5 |
Activated carbon |
Acetylation |
Acetic acid |
DAG + TAG |
91 |
62 |
[34] |
| 5. |
PW2-AC |
Activated Carbon |
Esterification |
Acetic acid |
Diacetin |
86 |
63 |
[35] |
| 6. |
SHTC |
D-glucose |
Esterification |
Acetic acid |
Mono acetin |
70 |
89 |
[28] |
| 7. |
SO 3H-C, |
Cellulose |
Acetalization |
Acetone |
Solketal |
80 |
100 |
[29] |
| 8. |
SO 3H-carbon |
Glycerol |
Esterification Acetylation |
Acetic acid, Acid anhydride |
TAG |
100 |
100 |
[34] |
| 9. |
SO 3H-glycerol-carbon |
Glycerol |
Acetylation |
Acetic acid |
TAG |
100 |
100 |
[40] |
| 10. |
[PrSO 3HN] [SO 3CF 3]/C |
Glucose |
Esterification |
Acetic acid Lauric acid |
TAG, MLG and DLG |
– |
– |
[32] |
| 11. |
GBCC |
Crude glycerol |
Esterification |
Acetic acid |
DAG + TAG |
99 |
88 |
[39] |
| 12. |
Sulfonated carbon |
Catkins from willow |
Esterification |
Acetic acid |
DAG |
98.4 |
54.5 |
[38] |
| 13. |
CX |
Glucose |
Acetylation |
Acetic acid |
DA + TA |
97 |
75 |
[26] |
| 14. |
Sulfonated Peanut Shell |
Peanut shell, |
Etherification |
Isobutylene |
DTBGs + TTBG |
100 |
92.1 |
[51] |
| 15. |
SCC-S |
Agroindustrial Wastes |
Etherification |
TBA |
DTBG + TTBG |
80 |
21.3 |
[54] |
| 16. |
Sulfonated carbon |
Sugar |
Etherification |
tert-butanol |
Mono and di-glyceryl |
– |
– |
[33] |
| 17. |
BCC-SF |
Coffee ground wastes |
Etherification |
TBA |
MTBG |
– |
42 |
[53] |
| 18. |
Au/AC |
Activated carbon |
Oxidation |
– |
DIHA |
72 |
18 |
[47] |
| 19. |
Au/MWCNT |
Multi-walled carbon nanotubes |
Oxidation |
– |
DIHA |
93 |
60 |
[47] |
| 20. |
10%Pd/C |
Activated carbon |
Oxidation |
– |
Lactic acid |
99 |
68 |
[48] |
| 21. |
5% Pt/C |
Activated carbon |
Oxidation |
– |
Lactic acid |
99 |
74 |
[48] |
| 22. |
0.5%Cu–1.0% Pt/AC |
Activated carbon |
Oxidation |
– |
Lactic acid |
80 |
69.3 |
[49] |
| 23. |
Pt/AC |
Activated carbon |
Oxidation |
– |
Lactic acid |
100 |
69.3 |
[50] |
| 24. |
10%HSiW/AC |
Activated carbon |
dehydration |
– |
Acrolein |
92.6 |
75.1 |
[56] |

Scheme 4.4 Steps for the formation of TAG using SO 3H-C derived from glycerol [30].
Malaika and co-workers have prepared SO 3H-modified carbon xerogels and spheres using glucose as a carbon source. These catalysts were explored for acetylation of glycerol and the optimum catalyst shows 75% selectivity for both diacetin (DA) and triacetin (TA), and almost complete glycerol conversion (About 97%). The results are comparable with commercially available ion exchange resin (Amberlyst 15) [26].
Ellis and coworkers have investigated the use of sulfonated carbon catalysts for transesterification and esterification reactions of glycerol. The active catalyst was synthesized by pyrolysis and sulfonation of the sugar char in a tube furnace. The reaction of glycerol with tert -butanol over sulfonated carbon leads to the formation of mono-glyceryl ethers isomers and di-glyceryl ether isomers [33].
Khayoon et al . have explored the potential of sulfated activated carbon catalysts (AC-SA5) for the transformation of glycerol into oxygenated fuel additives by glycerol acetylation. The AC-SA5 was prepared by functionalization of activated carbon with sulfuric acid by hydrothermal method. The catalyst shows 92% of the glycerol conversion into mono, di, and triacetyl glyceride with 38, 28, and 34% selectivity, respectively in a batch run. The catalyst shows good stability up to four consecutive steps [34].
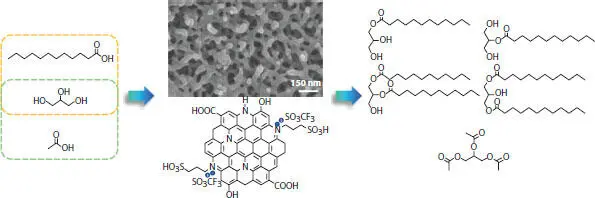
Figure 4.7 Selective glycerol esterification with acetic acid and lauric acid over [PrSO 3HN][SO 3CF 3]/C nanorods [32].
The glycerol transformation to monoacetin, diacetin, and triacetin over heteropolyacids modified activated carbon was investigated by Castanheiro and co-workers. The activity of the catalyst enhances with an increase in the loading of dodecatungstophosphoric acid (PW) on the surface of activated carbon. The maximum catalytic activity was observed for the catalyst with 4.9% loading. On further increasing the loading the activity decreases due to the blockage in the pores of activated carbon. The catalyst is stable up to three consecutive batch runs [35]. Wang et al . have explored the benefits of sulfonated hollow sphere carbon for acetylation of glycerol. Sulfonic groups modified hollow sphere carbon (HSC-SO 3H) has been prepared by carbonization of SiO 2core–shell polymer which was followed by elimination of the SiO 2core and functionalization with chlorosulfonic acid. The catalyst exhibits better activity for glycerol acetylation owing to the microporous structure which allows fast mass transfer during the reaction [36]. Rice husk-derived sulfonated carbon has been used as a potential catalyst for etherification and esterification of glycerol. The catalyst was synthesized by carbonization of rice husk followed by treatment with H 2SO 4. Glycerol esterification with acetic acid exhibits about 90% transformation to mono-, di-, and triglycerides with the selectivity of 11, 52, and 37% respectively after 5 h of reaction. The glycerol etherification with tert-butyl alcohol (TBA) shows a 53% conversion to di and tri tert-butylglycerol with 25% selectivity. The key role for promoting the activity of the catalyst was played by the Bronsted acidic sites and hydrophilicity which helps in preventing the catalyst deactivation [37]. The catalyst activity for various glycerol conversions depends upon the preparation methods. The effect of sulfonation on biomass-derived carbon catalyst was studied by Tao et al . [38]. The active catalyst was synthesized by sulfonation of carbonized catkins from the willow plant using different sulfonation conditions. It was found that the sulfonation conditions affect the density of acidic sites. The resulted catalyst exhibits almost complete transformation of glycerol in the presence of acetic acid into MAG, DAG, and TAG in 2 h at 393K. Compared to a similar kind of catalyst reported in the literature, this catalyst exhibits superior heat stability, recyclability, and water tolerance. Crude glycerol from biodiesel industries has been utilized for the conversion of glycerol into valuable products as well as for catalyst preparation. Hameed and coworkers have prepared solid acid catalyst by carbonization and sulfonation of biodiesel-derived crude glycerol. The catalyst shows almost complete conversion (99%) of glycerol with acetic acid into oxygenated fuel additives (DAG and TAG) and MAG. The catalyst is stable and can be used for up to seven cycles without appreciable loss in its performance. The catalyst has the potential for industrial conversion of crude glycerol at ambient reaction conditions [39]. Similarly, Karnjanakom and coworkers have prepared a sulfonated carbon-based catalyst by in situ carbonization and sulfonation. The ultrasound-assisted glycerol acetylation with acetic acid over this catalyst exhibits 100% selectivity towards the formation of TAG. The catalyst is highly stable and can be used effectively for ten repeated cycles. The presence of acidic sites along with ultrasound radiation contributes towards 100% selectivity for TAG [40].
Читать дальше














