Joseph J. Torres - Life in the Open Ocean
Здесь есть возможность читать онлайн «Joseph J. Torres - Life in the Open Ocean» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Life in the Open Ocean
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Life in the Open Ocean: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Life in the Open Ocean»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Life in the Open Ocean: The Biology of Pelagic Species
Life in the Open Ocean: The Biology of Pelagic Species
Life in the Open Ocean — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Life in the Open Ocean», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Sources: (a) Adapted from Uchida (1926); (b and c) Kaestner (1967), figure 5‐17 (p. 105); (d and e) Hyman (1940), figure 172 (p. 525); (f) Redrawn from Mayer (1910), plate 73.
The life histories of rhizostome medusae are typical of the Scyphozoa ( Figure 3.12c) in having a planula larva that settles to the bottom and forms a polypoid scyphistoma. Scyphistomae may strobilate to form ephyrae or may produce other scyphistomae by budding.
The rhizostomes are chiefly a tropical–subtropical group inhabiting shallow waters, though two genera, Rhizostoma and Stomolophus , are found in temperate climes and may even form blooms. Stomolophus nomurai forms huge blooms in the Sea of Japan at intervals (Mills 2001). Rhizostomes are generally quite large, with sizes ranging from 4 to 200 cm across the bell.
Genera include: Rhizostoma, Mastigias, Cassiopeia, Stomolophus, Cephea .
The Cubomedusae
The Cubomedusae, variously known as the box jellies, sea wasps, or fire medusae, comprise the Cnidarian class Cubozoa. The Cubomedusae were formerly considered to be an order in the Scyphozoa. Now they are considered their own class and comprise two orders, the Carybdeida and the Chirodropida. The group includes about 48 species found in tropical and subtropical latitudes.
Bells of the Cubomedusae are indeed cuboidal, with four flattened sides that are square when viewed from the exumbrellar side or in transverse section ( Figure 3.14a). The bell has a simple margin that bends inward to form a velarium ( Figure 3.14b). Tentacles arise from the four corners of the bell, either singly or in groups. The base of the tentacle(s), the pedalium, is a blade‐like structure that gives rise to a single tentacle in the family Carybdeidae and a palmate structure that gives rise to several tentacles in the family Chirodropidae ( Figure 3.14c and d). Below the pedalium, the tentacle is hollow and armed with rings of nematocysts.
A four‐sided manubrium leads to a simple, central stomach that is located at the apex of the sub‐umbrellar surface. The stomach differentiates into four gastric pockets that occupy the flattened sides of the umbrella.
The life history of the Cubomedusae is much like that of the hydromedusae and scyphomedusae with one important difference: the polypoid stage of the Cubomedusa does not strobilate. Rather, each polyp metamorphoses into an individual medusa. Arneson and Cutress (1976) described the development of Carybdea alata in Puerto Rican waters as proceeding from a released blastula stage to a swimming planula in 1 day, settlement of the planula in an additional 4 days, growth and maturation of the polyp for about 60 days, and the metamorphosis culminating in a liberated medusa taking an additional week, for a total of about 75 days for the entire process. Temperature during development was 26–29 °C.
Genera include: Carybdea, Tripedalia, Tamoya, Chirodropus, Chiropsalmis, and Chironex .
Foraging Strategies
The subject of foraging strategies covers a lot of ground, from diets and prey selectivity to models of encounter rates and predatory behavior (e.g. Gerritsen and Strickler 1977). Our chief concern is to describe what is known about feeding in medusae, including both diet (favorite foods) and elements of the feeding behavior itself. What is important to a weakly swimming, tentaculate, predator?
In most studies of feeding in medusae, hydromedusae are grouped together with scyphomedusae, siphonophores, and sometimes even ctenophores. Clearly, though differing in complexity and size, many elements of hunting will be highly similar between the hydromedusae, scyphomedusae, and cubomedusae because of their similar body shape. The section on foraging strategies will regard the medusae as a whole, though crossing taxonomic boundaries, as will the discussion of locomotion and energetics.
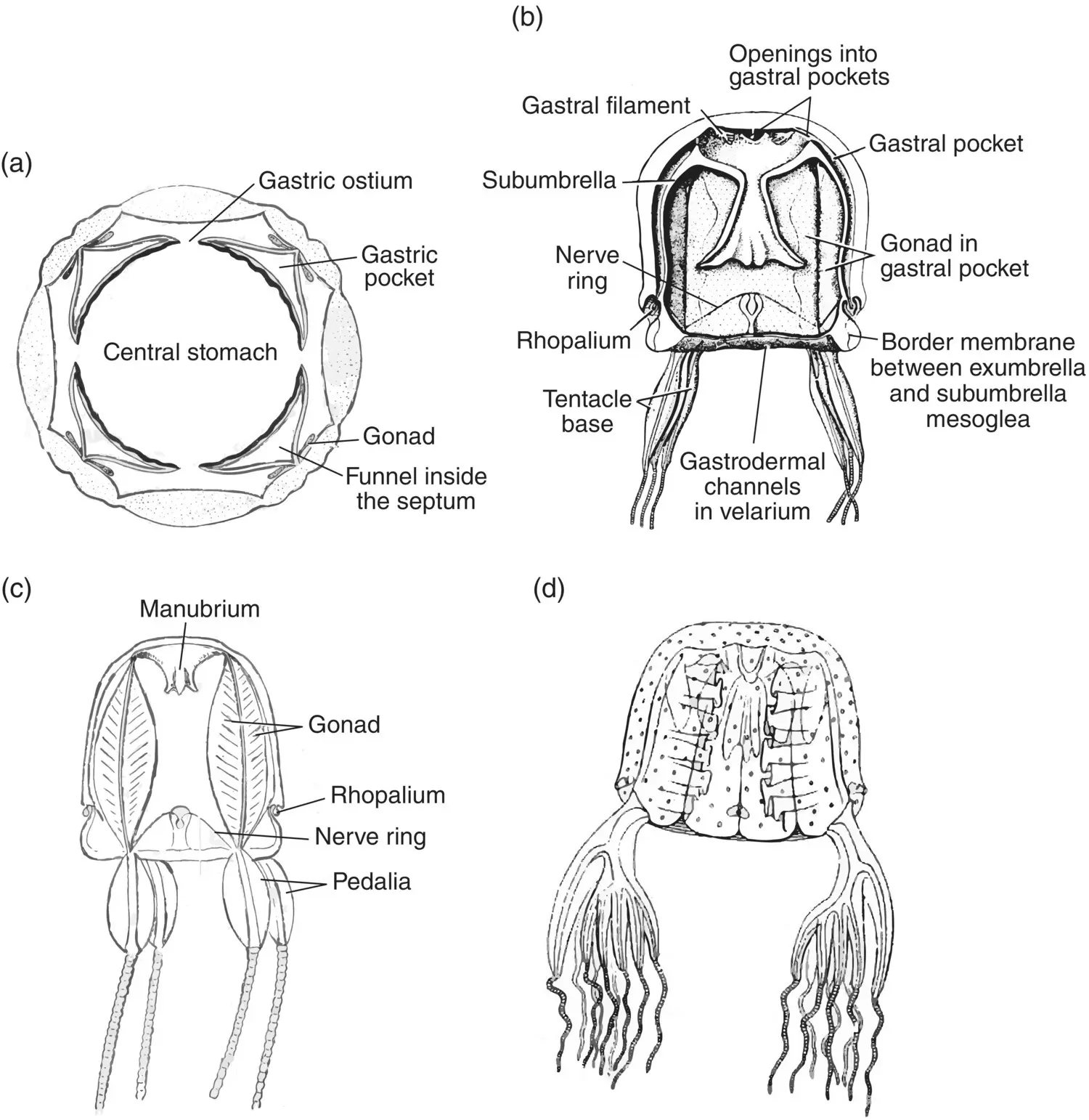
Figure 3.14 Cubomedusae. (a) Cross section through the bell of Carybdea ; (b) longitudinal section through Tripedalia ; (c) the Chirodropid medusa Carybdea . (d) The Chirodropid medusa Chiropsalmus .
Sources: (a–c) Conant (1898); (d) Redrawn from Mayer (1910), plate 47.
General Considerations
Perspective is important when evaluating the foraging behavior of medusae. Equipped with rudimentary sensory systems and limited locomotory capabilities, they forage in a profoundly three‐dimensional environment. Prey are captured on tentacles deployed in a stationary ambush or a slowly moving array as the animal swims forward. Stinging cells (nematocysts) on the tentacles paralyze the prey, which are then conveyed to the mouth and digested. Since both locomotion and the sensory field are quite limited, feeding success of a medusa will be determined by the number of its physical encounters with prey and the effectiveness of its tentacles in subduing the prey item.
In their mathematical model of predator–prey interactions in zooplankton, Gerritsen and Strickler (1977) assumed that the “encounter radius” of a predator was determined by its sensory system. In the case of medusae, it is determined by the volume enclosed within their tentacle array and the likelihood that a prey item once within the “kill zone” will contact a tentacle and be trapped (Madin 1988).
The Cnidae
The stinging organelles, or cnidae, that give the phylum Cnidaria its name are highly complex intracellular structures unique to the phylum. They are formed inside cells called cnidoblasts (Brusca and Brusca 2003), which are formed from interstitial cells in the epidermis and gastrodermis. The mature cnida in its cell is a cnidocyte. The majority of cnidocytes are located on the tentacles in small, blister‐like groups called “batteries” or in the epidermis of the oral region.
The cnida in its discharged state consists of a cup‐shaped basal capsule, a basal “shaft” or “butt” (Hyman 1940; Arai 1997), and ends in a long hair‐like tubule that is often invested with a spiny armor ( Figure 3.15). The venom used to subdue prey is either “injected” from a pore at the end of the tubule, much like a syringe works, or is introduced as a coating on the tubule, much like a poisoned dart. The trick to understanding the process of eversion or discharge is to understand how the cnida is coiled in its resting state. If you can imagine poking your finger into that of an empty rubber glove from the wrong side so that it turns “outside in”. you will have a good idea of how the cnida is coiled. In its resting state, it is literally turned outside in. When discharged, the pressure from within the cnida’s basal capsule causes it to evert, just as the finger on the rubber glove would pop back out to normal if you blew into the bottom of the glove. Cnidae can only be discharged once.
Their older name, nematocysts, is still very much in use, and the term cnidocyte then becomes nematocyte. In the newer terminology, only the stinging cnidae are termed nematocysts, to distinguish them from other types of cnidae that, for example, stick to prey (spirocysts) instead of envenomating them (Brusca and Brusca 2003).
Nematocysts, or cnidae, are considered to be “independent effectors”: their discharge is not governed by the nervous system of the medusa but will discharge when stimulated directly by prey contact. The nematocyst has a “lid” or operculum ( Figure 3.15) that covers the capsule and acts as a trapdoor. When the cnidocyte discharges, the operculum is flung open. The cnidocil, a bristle located next to the operculum, is believed to be the mechanoreceptor or “trigger” responsible for nematocyst discharge. Though the cnidocytes are considered independent effectors, their sensitivity threshold can be modified by the nutritional state of the medusa. A starved medusa will have a lower threshold for discharge than a well‐fed one.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Life in the Open Ocean»
Представляем Вашему вниманию похожие книги на «Life in the Open Ocean» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Life in the Open Ocean» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












