Flexible Thermoelectric Polymers and Systems
Здесь есть возможность читать онлайн «Flexible Thermoelectric Polymers and Systems» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Flexible Thermoelectric Polymers and Systems
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Flexible Thermoelectric Polymers and Systems: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Flexible Thermoelectric Polymers and Systems»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Comprehensive review of the rapidly evolving field of flexible thermoelectric polymers Flexible Thermoelectric Polymers and Systems
Flexible Thermoelectric Polymers and Systems
Flexible Thermoelectric Polymers and Systems — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Flexible Thermoelectric Polymers and Systems», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
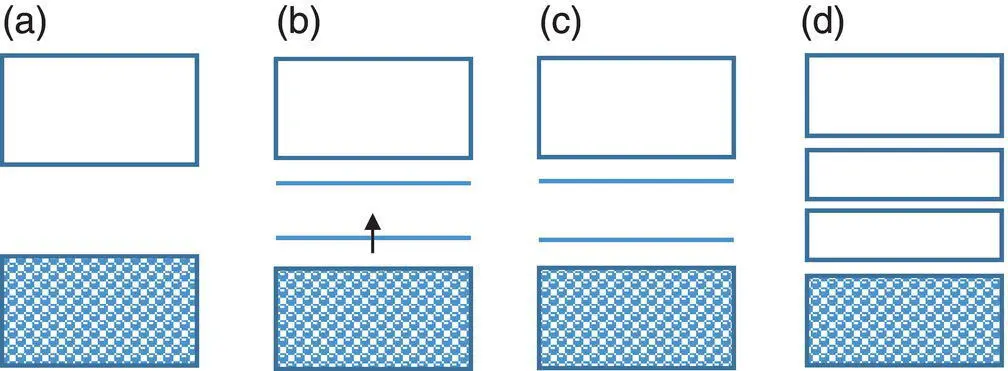
Figure 1.12 Band structures of conducting polymers with less symmetry in (a) neutral state, (b) low doping degree, (c) medium doping degree, and (d) high doping degree. There is one electron on the lower polaron level in (b), while no electron on the bipolaron levels and bipolaron bands in (c) and (d).
1.1.5.2 Charge Carrier Mobility
The conduction electrons in metals are the valence electrons of metal atoms. The conduction electrons can be scattered by the lattice consisted of metal cations, and the lattice scattering lowers the electron mobility. Because of the much higher ion concentration in metals than in inorganic semiconductors, metals usually have a much lower charge carrier mobility than inorganic semiconductors. The charge carrier mobility of inorganic semiconductors is mainly affected by impurities, defects, and lattice vibration. A doping atom can be considered as an impurity, and it forms a scattering center for the charge carriers. As a result, doping lowers the charge carrier mobility. Therefore, the charge carrier mobility of inorganic semiconductor and conducting polymers usually decreases with the increasing doping level.
Different from metals and inorganic semiconductor that can be prefect crystals, a conducting polymer cannot have 100% crystallinity. There are both amorphous and crystalline domains in conducting polymers ( Figure 1.13). The amorphous domain has a conductivity much lower than that of the crystalline domain. The charge transports include intrachain, interchain, and inter‐domain processes. The interchain charge transport is usually the dominant process. Thus, the charge carrier mobility of conducting polymers strongly depends on the crystallinity. High crystallinity can facilitate the interchain charge transport, and thus give rise to high charge carrier mobility. A conducting polymer with the same chemical composition can have conductivities spanning several orders by magnitude.
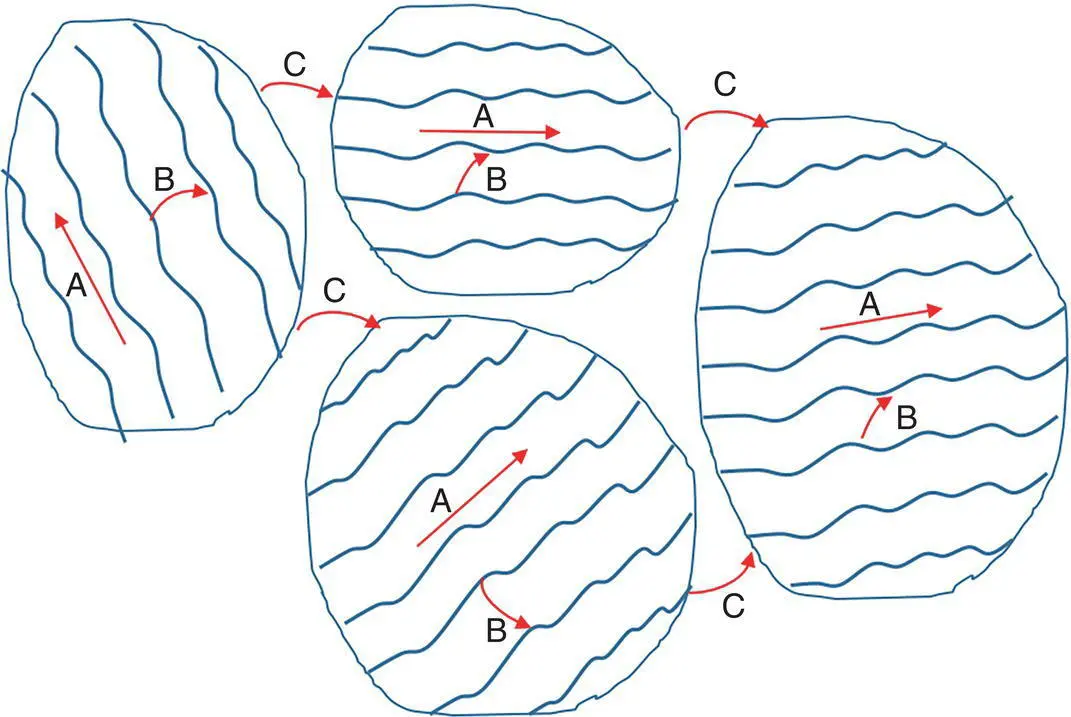
Figure 1.13 (a) Intrachain, (b) interchain, and (c) inter‐domain charge transports in conducting polymers.
The crystallinity of some conducting polymers can be increased through post treatment. As a result, the conductivity can be enhanced by several orders of magnitude. When a chemical is used for the post treatment, the post treatment method is usually called “secondary doping.” Secondary doping has been extensively reported for solution‐processable conducting polymers including polyaniline and PEDOT:PSS [15–19]. Polyaniline doped with a large counter anion like camphorsulfonic acid (HCSA) can be dispersed in organic solvent such as m ‐cresol and chloroform [20]. The solvent significantly affects the crystallinity and the conductivity of polyaniline:HCSA (PANi:HCSA) because it can affect the conformation of the polymer chains in solution. When a PANi:HCSA film is prepared from chloroform, its conductivity is only 10 −1S cm −1, while it can be >100 S cm −1when it is obtained from m ‐cresol. This difference in conductivity is attributed to the effect of the solvents on the crystallinity of polyaniline. As shown in Figure 1.14, there is a strong absorption in the infrared range for the PANi:HCSA film cast from its m ‐cresol solution [15]. This suggests a high crystallinity and the delocalization of the charge carrier. Instead, only a band appears in the near infrared (NIR) range when chloroform is used as the solvent. This is consistent with the low crystallinity.
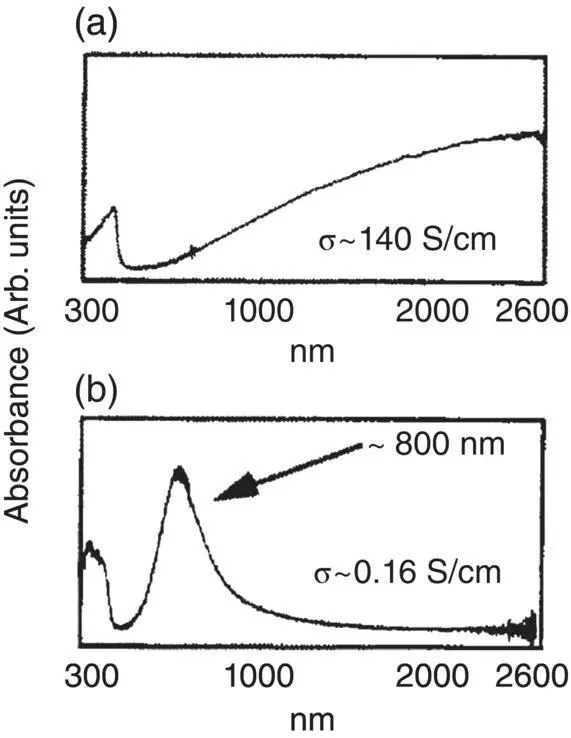
Figure 1.14 UV–visible–NIR absorption spectra of PANi:HCSA films casted from solutions with (a) m ‐cresol or (b) chloroform as the solvent, respectively.
Source: MacDiarmid and Epstein [15]. © Elsevier.
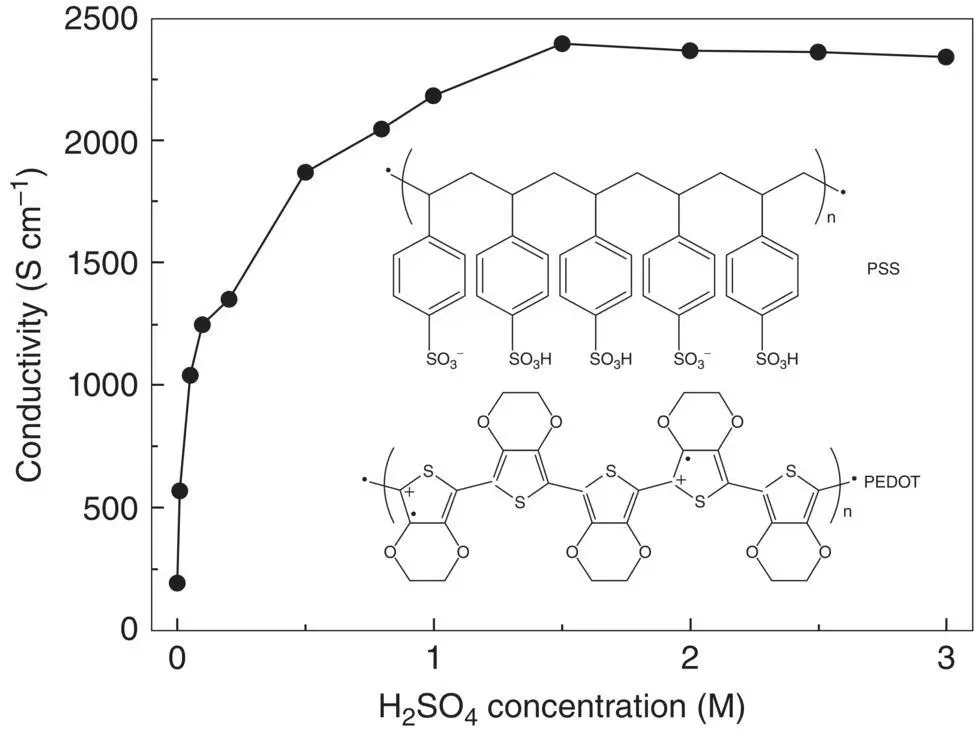
Figure 1.15Conductivity of PEDOT:PSS after secondary doping with H 2SO 4.
Source: Xia et al. [21]. © John Wiley & Sons.
A couple of secondary doping methods were also reported for PEDOT:PSS. PEDOT:PSS can be dispersed in water [17–19]. The conductivity of an as‐prepared PEDOT:PSS film is only 10 −1S cm −1, and it can be significantly enhanced by secondary doping. The chemicals for the secondary doping can be organic solvents like dimethyl sulfoxide (DMSO), ethylene glycol (EG) and even methanol, cosolvent of water and organic solvents, aqueous or organic solutions, ionic liquids, and acids. Particularly, the conductivity of PEDOT:PSS can be enhanced from 10 −1S cm −1to higher than 3000 S cm −1through a post acid treatment [21–24]. The conductivity enhancement is ascribed to the partial removal of the insulating PSSH from PEDOT:PSS and the conformational change of PEDOT from coil to expanded‐coil or linear structure. Less PSSH chains and linear PEDOT conformation can lead to high crystallinity and thus high conductivity.
Mechanical stretching can orient polymer chains along the tensile direction and thus increase the crystallinity. It has been used to increase the conductivity of conducting polymers as well [25, 26]. For example, it can increase the conductivity of polyacetylene up to 10 5S cm −1.
1.1.5.3 Temperature Dependence of Conductivity
Temperature dependence of the conductivity is often used to study the charge transport mechanism because metals and semiconductors have different temperature dependences of their conductivity. Conducting polymers are considered as disordered systems. Their charge transport is dominated by interchain charge hopping, that is different from those of both metals and semiconductors.
1.1.5.3.1 Metals
The temperature dependence of the conductivity is related to the charge transport mechanism. Varying the temperature does not affect the charge carrier density but the charge carrier mobility of metals. As the lattice vibrations become stronger at higher temperature, the scattering of the conduction electrons by lattice vibrations becomes more significant at higher temperature. This lowers the charge carrier mobility. The conductivity of metals thus decreases with the increasing temperature. There are two empirical formulas for the temperature dependence of the resistance of metals. One is the power law form,
(1.15) 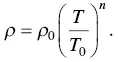
The resistivity ( ρ ) at temperature ( T ) is related to the resistivity ( ρ 0) at the reference temperature ( T 0). The resistivity linearly increases with temperature in another form, and the temperature effect is characterized by the temperature coefficient of resistivity ( α 0),
(1.16) 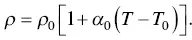
In both forms, the resistivity of metals increases with the increasing temperature.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Flexible Thermoelectric Polymers and Systems»
Представляем Вашему вниманию похожие книги на «Flexible Thermoelectric Polymers and Systems» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Flexible Thermoelectric Polymers and Systems» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












