Cheanyeh Cheng - Enzyme-Based Organic Synthesis
Здесь есть возможность читать онлайн «Cheanyeh Cheng - Enzyme-Based Organic Synthesis» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Enzyme-Based Organic Synthesis
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Enzyme-Based Organic Synthesis: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Enzyme-Based Organic Synthesis»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
An insightful exploration of an increasingly popular technique in organic chemistry Enzyme-Based Organic Synthesis
Enzyme-Based Organic Synthesis
Enzyme-Based Organic Synthesis
Enzyme-Based Organic Synthesis — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Enzyme-Based Organic Synthesis», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
To shift the reaction equilibrium to the product side of the transamination reactions catalyzed by transaminase, a conventional strategy that has been commonly used is to provide an excessive amount of the amino donor. Scheme 3.1illustrates an example in which an excess amount of isopropyl amine was applied to push the transamination of acetophenone to 1‐phenylethylamine toward complete conversion and an enantiomeric excess of >99% [3]. In addition to the method of using excessive cosubstrate for shifting the equilibrium toward the product side, other methods such as removal of product or coproduct by vaporization, extraction, autodegrading smart coproducts, and biochemical cascade reactions were also utilized in many cases [1].

Scheme 3.1 The transamination between acetophenone and excessive isopropyl amine with transaminase.
Source: Truppo et al. [3].
Another smart synthesis strategy is the combination of transaminase with other biocatalysts to form enzymatic cascade reactions that have been used to efficiently produce unnatural amino acids, optically pure amines, imines, secondary amines, and amides, or for the amination on the –OH or –CH 3functional group, or for the amines with two stereocenters. The enzymatic cascade reactions in organic synthesis give the advantages of shortening reaction routes, avoiding unstable or toxic intermediate, increasing the atom efficiency, and reducing the amount of wastes [1]. Most importantly, the enzymatic cascade reactions involving transaminases show prominent potential for many industrial applications [2].
There have been many examples to demonstrate the use of ω‐transaminase (ω‐TA) for producing unnatural amino acids such as L‐ tert ‐leucine and L‐phosphinothricine [4]. One of the many examples employing coupled enzyme reactions for synthesizing the unnatural amino acids was the production of the unnatural L‐homoalanine from L‐threonine by combining a threonine deaminase (TD) with the ω‐TA and using an one‐pot reaction process ( Scheme 3.2) that resulted a 91% conversion [5]. The source of TD was from a cloned and overexpressed Escherichia coli and the ( S )‐specific ω‐TA was from Paracoccus denitrificans . The formation of N ‐benzylacetamide from benzaldehyde through an efficient one‐pot biocatalytic amine transaminase/acyl transferase cascade using methyl acetate as acyl donor is shown in Scheme 3.3[6].
The conversion of the amide product in this enzymatic cascade reaction was up to 97% with an acyl transferase (AcT) from Mycobacterium smegmatis and an amine transaminase from Silicibacter pomeroyi .

Scheme 3.2 Enzyme cascade reactions for the conversion of L‐threonine to L‐homoalanine using a one‐pot reaction process.
Source: Modified from Park et al. [5].
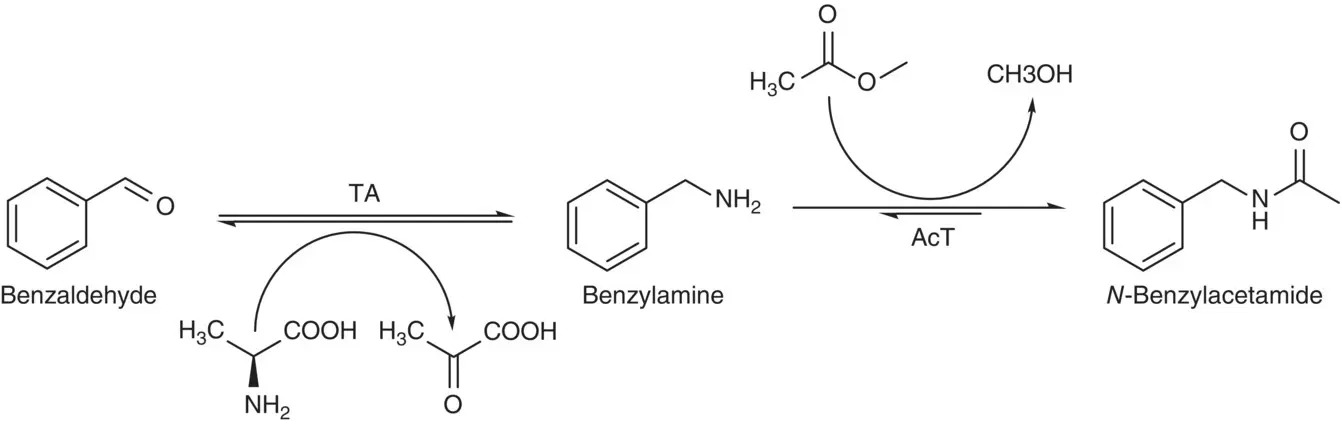
Scheme 3.3 The formation of N ‐benzylacetamide from benzaldehyde through an efficient one‐pot biocatalytic amine transaminase/acyl transferase cascade.
Source: Based on Land et al. [6].
Instead of compounds with one stereocenter, chiral amino alcohols with two stereocenters can be synthesized by the enzymatic cascade reactions without using protection step for the first stereocenter before the production of the second stereocenter. Scheme 3.4shows an example of the enzymatic cascade route toward all four diastereomers of 4‐amino‐1‐phenylpentane‐2‐ol started from a racemic 1,3‐hydroxy ketone [7].
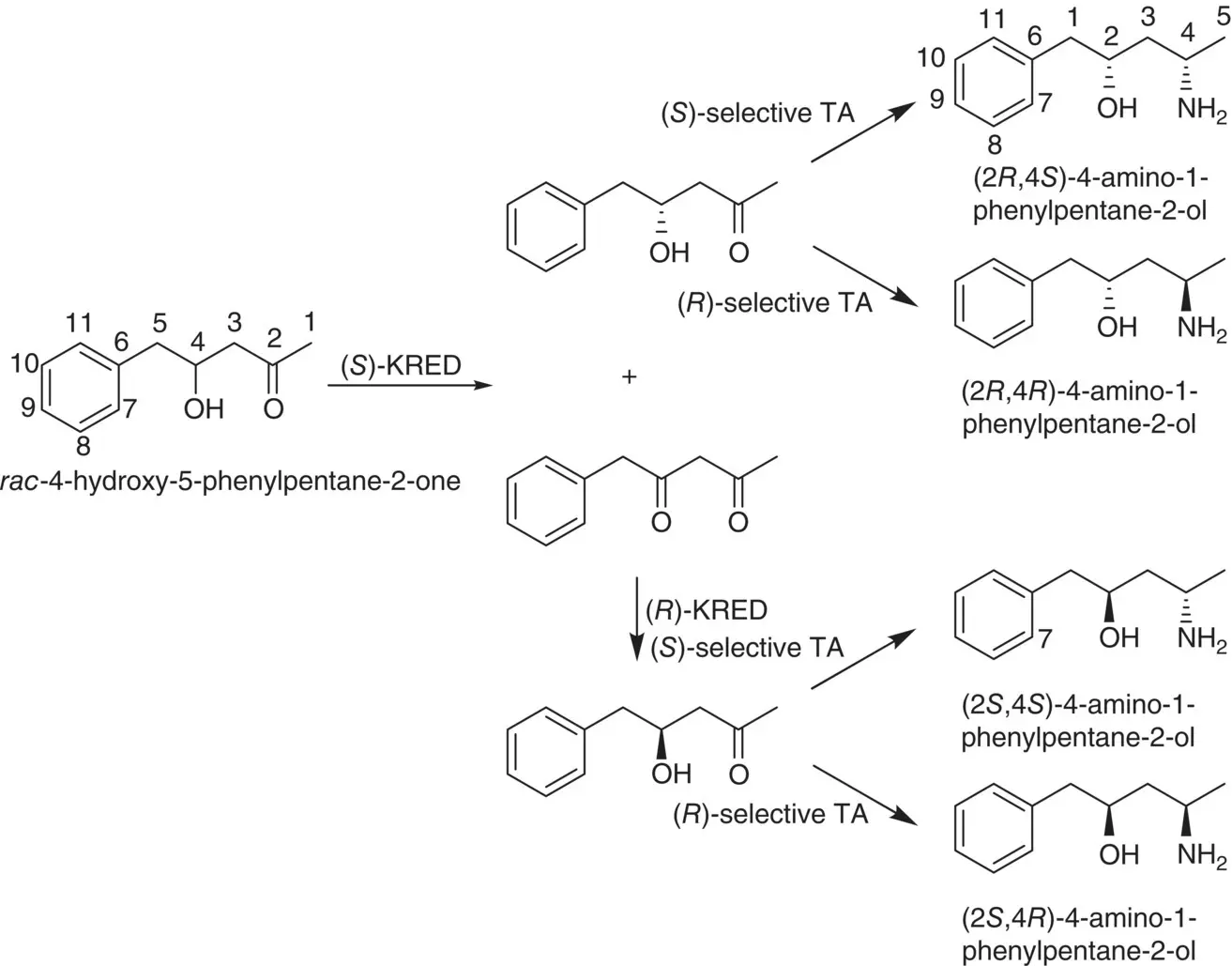
Scheme 3.4 The enzymatic cascade route toward all four diastereomers of 4‐amino‐1‐phenylpentane‐2‐ol from a compound with two stereocenters.
Source: Modified from Kohls et al. [7].
The first synthesis step of these four 1,3‐amino alcohol diastereomers was through the kinetic resolution for a racemic 1,3‐hydroxy ketone by applying using an ( S )‐selective keto reductase (KRED) to regioselectively provide the optically pure ( R )‐hydroxyl ketone (86% e.e .) and the corresponding diketone. Further transamination of the ( R )‐hydroxyl ketone using either an ( R )‐ or an ( S )‐selective TA yields the (2 R ,4 R )‐ and (2 R ,4 S )‐1,3‐amino alcohol diastereomers.
The diketone was then used to prepare the remaining two diastereomers, (2 S ,4 S )‐ and (2 S ,4 R )‐1,3‐ amino alcohol, by first converting the diketone to ( S )‐1,3‐hydroxy ketone and subsequently using either an ( R )‐ or an ( S )‐selective TA to stereoselective amination of the ( S )‐1,3‐hydroxy ketone to (2 S ,4 R )‐1,3‐amino alcohol and (2 S ,4 S )‐1,3‐amino alcohol, respectively.
The industrial applications of enzymatic cascade reactions can be demonstrated by the in vitro biosynthesis of the nylon‐6 monomer 6‐aminohexanoic acid (hydrolyzed ε‐caprolactam) from cyclohexanol as illustrated by Scheme 3.5[8]. In addition to transaminase, this enzymatic cascade route additionally involves a Baeyer–Villiger monooxygenase (BVMO) and an esterase that all these biocatalysts can function independently in a one‐pot synthesis under mild conditions. Two cofactor self‐sufficient cascade modules are involved in this route. One is for the production of ε‐caprolactone from cyclohexanol, the other is the subsequent production of 6‐aminohexanoic acid. This biosynthesis route thus shows advantages over chemical approaches that require sophisticated transition‐metal catalysts and high temperature and pressure.
Generally, the asymmetric amination of prochiral ketones with an amine donor employing ω‐transaminases (ω‐TAs) is performed in aqueous medium. Since most ketones are lipophilic with only moderately soluble in aqueous solution, the transformations involving ω‐TAs require the addition of an organic cosolvent, and enzyme engineering might be necessary to let it stand the harsher reaction conditions. In addition, the amination of prochiral ketones with ω‐TAs is commonly employed at pH < 10; the product amines are generally protonated necessitating the basification of the reaction mixture and the extraction with organic solvents. As a result, it would be desirable to employ ω‐TAs for the transamination exclusively in organic solvents. Mutti et al. employed nine different lyophilized crude cell‐free extracts of ( R )‐selective and ( S )‐selective ω‐TAs for the asymmetric amination of ketones in organic solvents with 2‐propylamine as amine donor. The best enzyme activity for the transamination was found for methyl tert ‐butyl ether (MTBE) at a water activity of 0.6 that allowed excellent stereoselectivity of optically pure amines ( e.e . > 99%) and a conversion rate > 99% [9].
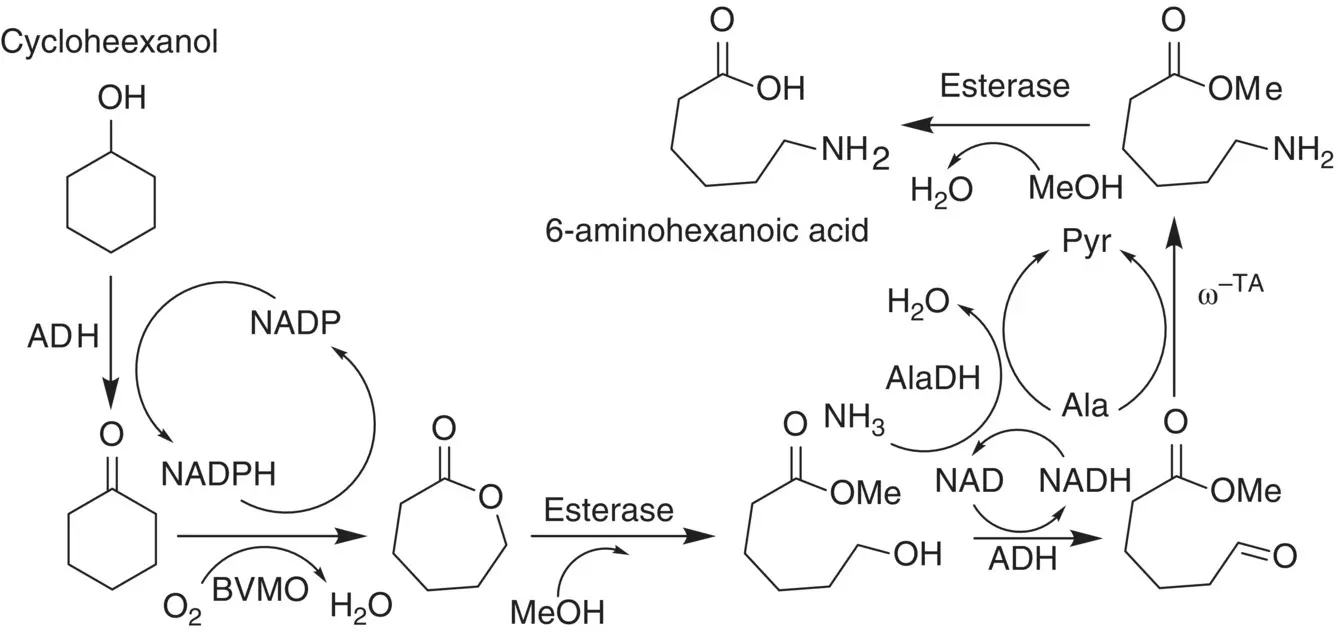
Scheme 3.5 The oxidation/transamination cascade reactions for the biosynthesis of 6‐aminohexanoic acid from cyclohexanol.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Enzyme-Based Organic Synthesis»
Представляем Вашему вниманию похожие книги на «Enzyme-Based Organic Synthesis» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Enzyme-Based Organic Synthesis» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












