Dennis D. Miller - Food Chemistry
Здесь есть возможность читать онлайн «Dennis D. Miller - Food Chemistry» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Food Chemistry
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Food Chemistry: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Food Chemistry»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
A manual designed for Food Chemistry Laboratory courses that meet Institute of Food Technologists undergraduate education standards for degrees in Food Science Food Chemistry: A Laboratory Manual
Food Chemistry: A Laboratory Manual
Food Chemistry — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Food Chemistry», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Answers to Problem Set
1 Volume of CO2 = 99 ml
2 Fast‐acting acids dissolve rapidly, slow‐acting acids dissolve more slowly.
3 Bicarbonate has a high pKa, which means it will not dissociate until the pH is high.
4 Double‐acting powders allow release of some CO2 prior to baking which helps improve batter viscosity.
5 1.25 lb MCP.
6 Normality of vinegar = 0.833 N. It will take 12 ml vinegar to neutralize 100 ml of 0.1 N NaOH.
7 143 ml vinegar.
8 Two equations may be written: Monoprotic: Diprotic: The published value of 80 lies between 67 and 133. This suggests that the sodium bicarbonate reacts nearly completely with the first H on the H2PO4 − but only partially with the second, i.e. the diprotic reaction above does not go to completion.
9 1.72 l CO2.
10
11
12
13 A lactone is an ester formed from the reaction of a carboxyl group and an alcohol group on the same molecule. Glucono‐delta‐lactone slowly hydrolyzes in water to form gluconic acid.
14 22.9%.
15 50 ml.
3 Properties of Sugars
3.1 Learning Outcomes
After completing this exercise, students will be able to:
1 Draw structures of common reducing and nonreducing sugars.
2 Explain the difference between a hemiacetal and an acetal.
3 Distinguish between reducing and nonreducing sugars experimentally.
3.2 Introduction
Sugars are polyhydroxylated aldehydes or polyhydroxylated ketones ( Figure 3.1). Thus, they participate in reactions characteristic of alcohols and aldehydes or ketones. Please review the sections in your organic chemistry textbook that describe reactions for alcohols, aldehydes, and ketones.
Recall that alcohols react reversibly with aldehydes or ketones to form hemiacetals or hemiketals. When the alcohol and carbonyl groups are on the same molecule, as is the case with sugars, a cyclic or ring structure is formed ( Figure 3.2).
Note that hemiacetals contain a carbon atom bonded to an –OH group and an –O–R group. Hemiacetals are relatively unstable. In aqueous solution, the open and closed ring forms are both present in equilibrium. Thus, sugars like glucose participate in reactions characteristic of aldehydes even though the predominant form is the hemiacetal.
Sugars containing the hemiacetal group are called reducing sugars because they are capable of reducing various oxidizing agents. Several well‐known assays, based on this tendency to oxidize, have been developed for detecting reducing sugars. These include the Tollen's test (sugars are mixed with Ag +in aqueous ammonia solution), the Fehling's test (sugars are mixed with Cu 2+in aqueous tartrate solution), and the Benedict's test (sugars are mixed with Cu 2+in aqueous citrate solution). When mixed with these solutions, reducing sugars are oxidized causing a reduction in the valence of the metal ion. In the Tollen's test, a shiny mirror of elemental silver (Ag 0) forms on the inside surface of the test tube. In the Fehling's and Benedict's tests, Cu 2+is reduced to Cu 1+which reacts with water to form reddish brown cuprous oxide. Benedict's reagent is used in some of the “sugar sticks” diabetics use to test their urine for spilled sugar. The following chemical equation describes the Benedict’s test [1]:
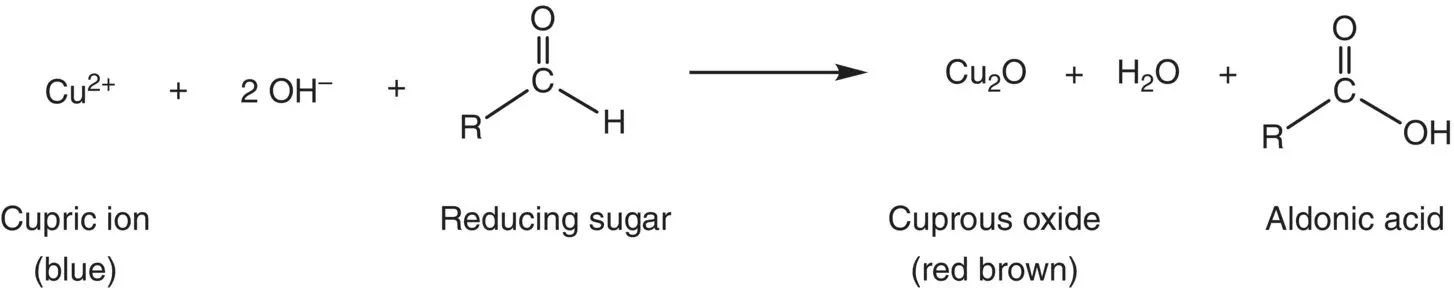
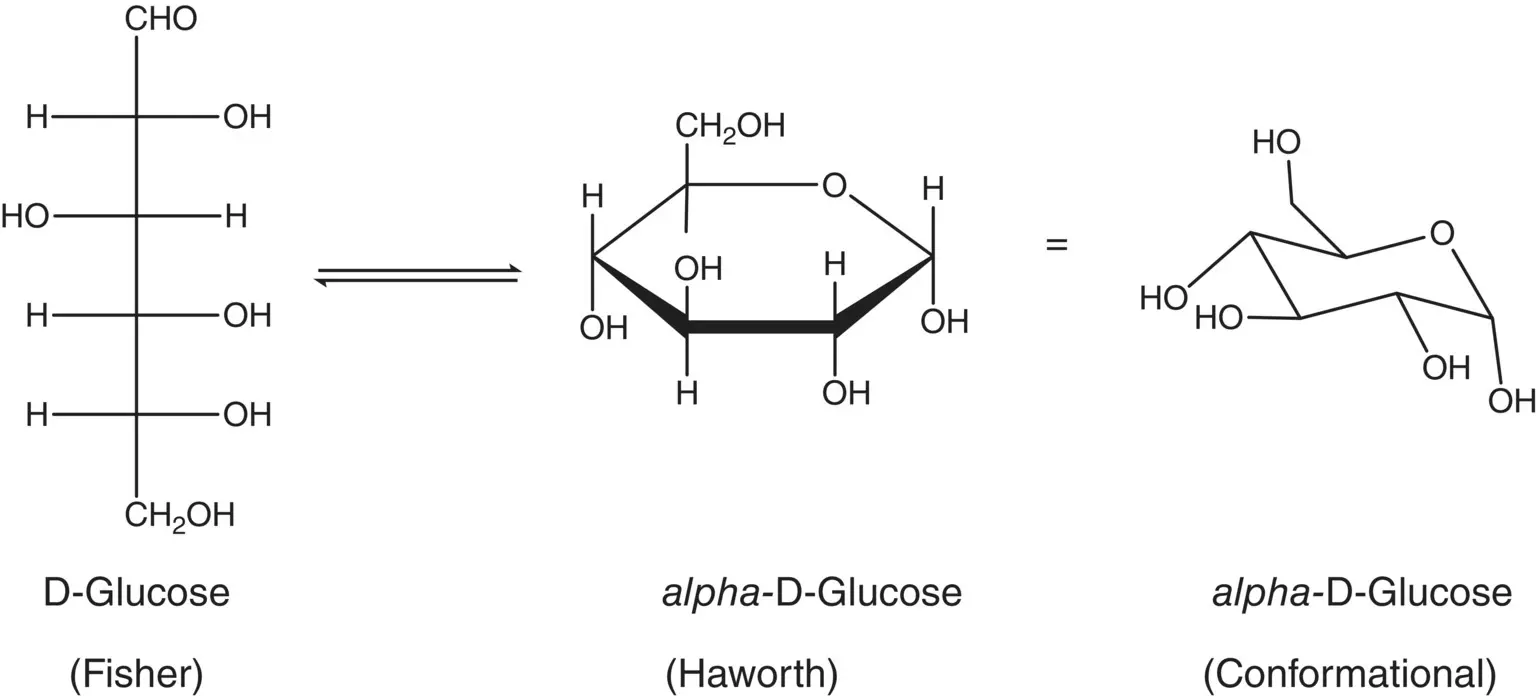
Figure 3.1 Three representations of the structure of glucose, a polyhydroxylated aldehyde.
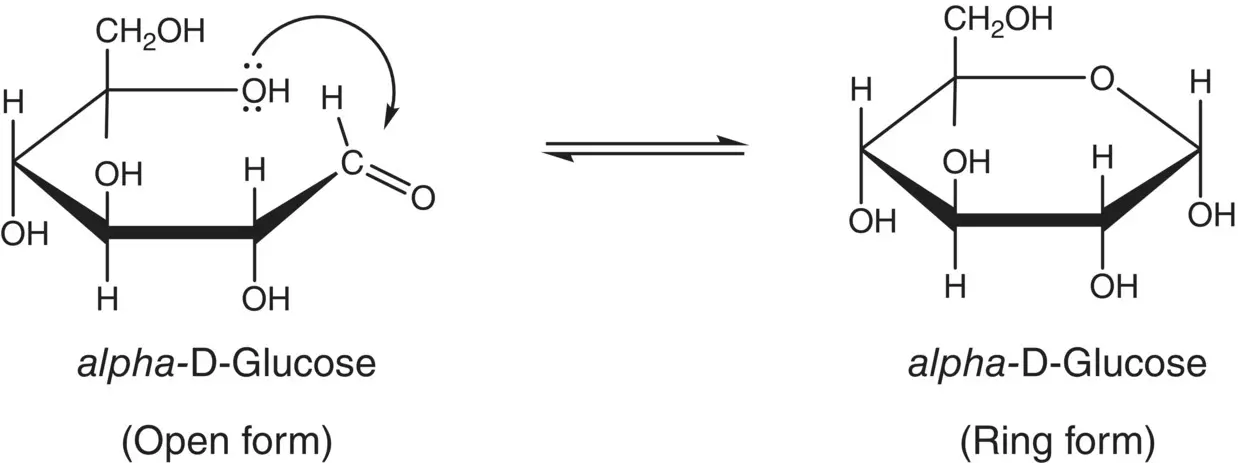
Figure 3.2 Balanced equation showing the nucleophilic attack of the C‐5 hydroxyl oxygen of glucose on the carbonyl carbon of the same molecule to form a hemiacetal.
When conditions are right, hemiacetals can react with alcohols to form acetals. For example, glucose in the hemiacetal form might react with fructose to form the acetal better known as sucrose ( Figure 3.3). Carbohydrate acetals are called glycosides.
Recall that acetals contain a carbon bonded to two –O–R groups. Unlike hemiacetals, acetals are relatively stable in aqueous solution and do not readily split into the hemiacetal and alcohol forms. However, they may be split into the alcohol and hemiacetal by acid‐catalyzed hydrolysis.
3.3 Apparatus and Instruments
1 Test tubes
2 Volumetric flasks, 50 ml
3 Hot plate
4 Large beaker, 600 ml
5 Water bath, 37 °C
6 Water bath, boiling
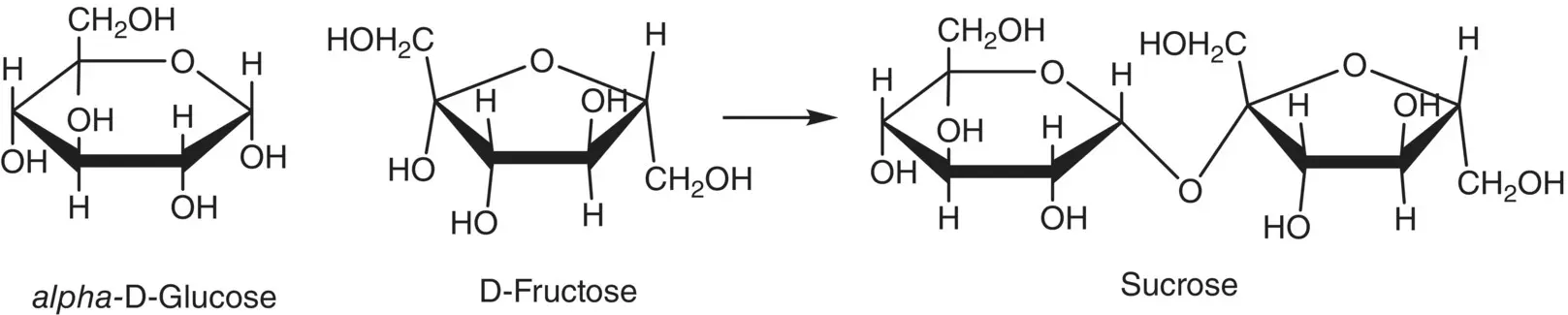
Figure 3.3 The formation of sucrose, a 1,2‐glycoside, from glucose and fructose.
3.4 Reagents and Materials
1 Crystalline glucose, fructose, sucrose, lactose, and sorbitol
2 HCl, 0.5 N
3 NaOH, 1.0 N
4 Benedict's solution (CuSO4 in citrate/carbonate buffer) [2].To prepare Benedict's solution: Dissolve, with stirring, 17.3 g sodium citrate and 10 g Na2CO3 in 80 ml distilled water. Dissolve 1.73 g CuSO4 .5H2O in 10 ml water. Add the CuSO4 solution to the citrate/carbonate solution. Transfer to a 100 ml volumetric flask. Dilute to 100 ml and mix well.
5 Starch solution (3% soluble starch in water)
6 Amylase solution (~1% amylase in water)
3.5 Procedures
1 Transfer 3 ml starch solution to each of two test tubes.
2 Add 5 drops of amylase solution to one of the tubes from Step 1 and incubate both tubes for 15 minutes in a 37 °C water bath.
3 Prepare solutions of glucose, fructose, sucrose, lactose, and sorbitol (50 ml of each, 0.1 mol l−1, in water).
4 Prepare 50 ml of 0.1 mol l−1 sucrose in 0.5 N HCl.
5 Transfer 5‐ml aliquots of the sucrose‐HCl solutions (from Step 4) to two test tubes.
6 Heat one of the test tubes from Step 5 in a boiling water bath for 10 minutes, cool. Keep the other at room temperature. Neutralize the HCl in the tubes by adding 1 ml of 1.0 N NaOH to each tube.
7 Transfer 3 ml of each of the solutions, including the starch solutions and the heated and unheated sucrose solutions, into separate test tubes.
8 Add 8 drops of Benedict's solution to each tube (including the starch solutions and a water blank). Mix well.
9 Place tubes in boiling water bath for three minutes.
10 Remove tubes from bath and observe color and appearance of solutions.
3.6 Study Questions
1 Draw the structure of each of the sugars you tested. Label them reducing or nonreducing.
2 What structure is characteristic of reducing sugars? Explain.
3 Compare your results from the 3 sucrose solutions (in water, in HCl, in HCl with heating). Explain any differences.
4 Compare the structures of glucose and sorbitol (a sugar alcohol).
5 Fructose is not an aldose, yet it is a reducing sugar. Explain. (Hint: Remember that tests for reducing sugars are conducted in alkaline solution and recall the concept of keto‐enol tautomerism.)
6 Name and draw the structures of 5 glycosides that may be present in foods.
7 Are all disaccharides nonreducing sugars? Explain.
8 Is starch reducing? Explain. Is starch treated with amylase reducing? Explain.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Food Chemistry»
Представляем Вашему вниманию похожие книги на «Food Chemistry» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Food Chemistry» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












