Polar Organometallic Reagents
Здесь есть возможность читать онлайн «Polar Organometallic Reagents» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Polar Organometallic Reagents
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Polar Organometallic Reagents: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Polar Organometallic Reagents»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Polar Organometallic Reagents
Polar Organometallic Reagents
Polar Organometallic Reagents — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Polar Organometallic Reagents», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
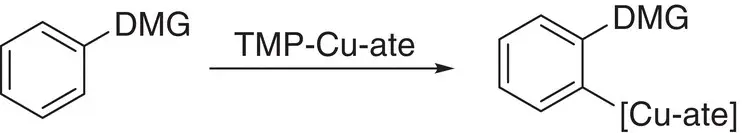
Scheme 1.34 A generalized view of directed ortho ‐cupration.
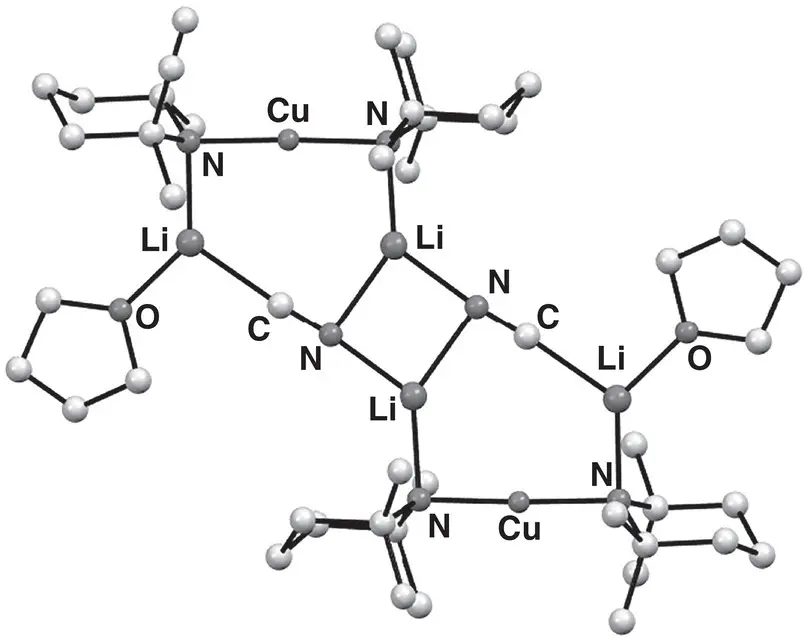
Figure 1.22 Molecular structure of Lipshutz cuprate dimer [(TMP) 2Cu(CN)Li 2(THF)] 2 159 2.
Source : Adapted from Usui et al. [213].
Structural work has been integral to the evolution of directed cupration. The concept of organo(amido)cuprate bases can be viewed as emerging from the confluence of organocuprate chemistry and the (at the time) relatively new and evolving field of synergic base chemistry. From the perspective of cuprate chemistry, it had been recognized for some time that the replacement of an organyl group with a non‐transferable ligand [212], to give a heterocuprate could (i) reduce wastage of valuable organic groups and (ii) significantly improve reagent stability. Several classes of non‐transferable ligand have been investigated [216]. However, amido groups have offered the most compelling combination of excellent stabilizing properties and relative ease of access from commercial reagents [217]. Of course, the ability of the sterically congested amido ligand TMP to act as a potent kinetic base in directed metalation has already been discussed in this chapter in the context of highly successful synergic metalating reagents such as 1[174]. The combination of these factors led to organo(TMP)cuprates being conceived as viable bases for directed cupration. Their successful application to the elaboration of functionalized aromatics is described in the preceding section [213] and is related in detail in Chapter 8. Most relevant to the work of an applied vein, the combination of LTMP with CuCN in THF was argued to form cyanide‐containing Lipshutz bis(amido)cuprate complex (TMP) 2Cu(CN)Li 2(THF) 159, this system demonstrating excellent reactivity in directed ortho ‐cupration. As a part of the same study, 2 : 1 reaction of LTMP with CuCN in the presence of THF indeed enabled the isolation of, and X‐ray diffraction studies on, this Lipshutz cuprate. Data revealed a dimer composed of individually 7‐membered metallacycles that associate by forming a central Li 2N 2ring ( Figure 1.22). The inclusion of cyanide as a bridge between multiple Li centres without any discernable interaction with Cu was significant since it provided further important evidence (backing up prior theoretical work) [160, 161] for the absence of higher‐order structures in cyanocuprates. This motif was subsequently found to apply to structures incorporating a range of inorganic anions that led to the development of the expression ‘Lipshutz‐type’ cuprates for the recently reviewed family of complexes (TMP) 2Cu(X)Li 2(X = inorganic anion ≠ CN, see below for specific examples) [218].
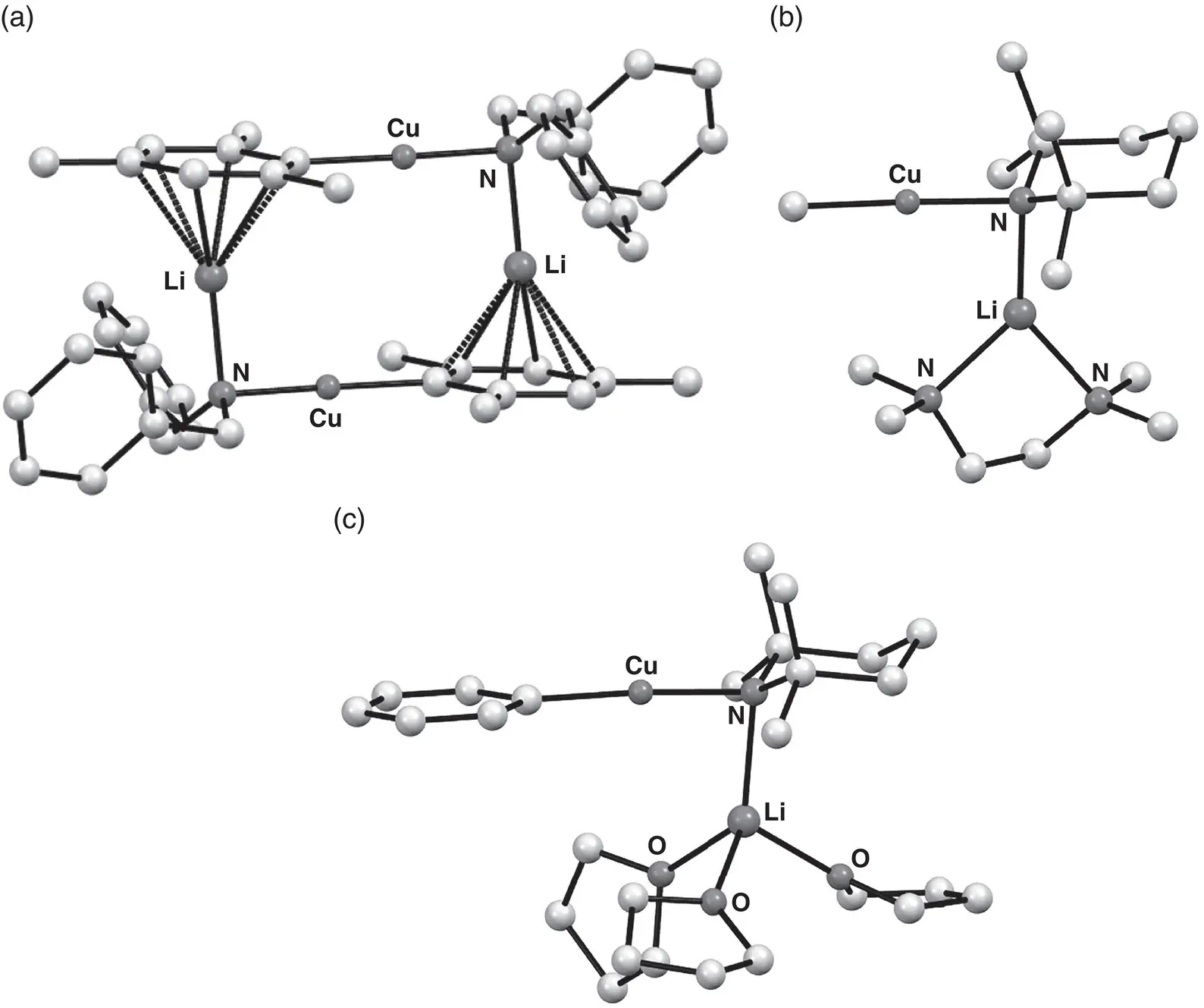
Figure 1.23 Molecular structures of organoamidocuprates (a) [MesCu(NBn 2)Li] 2 160 2, (b) MeCu(TMP)Li(TMEDA) 161and (c) PhCu(TMP)Li(THF) 3 162.
Sources : Adapted from Davies et al. [219]; Haywood et al. [220].
Reports on the structures of organo(amido)cuprates emerged around the same time as the inception of directed ortho ‐cupration. The combination of mesitylcopper with dibenzylamidolithium in toluene led to the isolation of Gilman heterocuprate MesCu(NBn 2)Li 160(Bn = benzyl), which revealed a head‐to‐tail dimer in the solid state ( Figure 1.23) [219]. In this case, Li salts and donor solvents were excluded during cuprate formation. On the other hand, in situ cuprate synthesis using mixtures of organolithium and amidolithium reagents in combination with CuCN offered the possibility of LiCN inclusion in the solution and/or solid‐state structures. In such cases, however, Gilman organo(amido)cuprates MeCu(TMP)Li(TMEDA) 161and PhCu(TMP)Li(THF) 3 162( Figure 1.23) that did not include LiCN were isolated and analyzed in the solid‐state. Both species preferred to form solvated monomers [220]. However, the inactivity of these isolated cuprates in directed ortho ‐metalation was in stark contrast to the behaviour of the in situ preparations, suggesting LiCN involvement to be likely in solution. This hypothesis was supported by DFT calculations, which suggested a facile equilibrium between Lipshutz and Gilman organo(amido)cuprates in solution.
Similar patterns of reactivity have been seen for bis(amido)cuprate preparations based on CuI, whereby Gilman cuprate 156(actually a dimer – see below) proved an ineffective base, whereas Lipshutz‐type (TMP) 2Cu(I)Li 2(THF) 163performed much better ( Scheme 1.35and Figure 1.24) [221]. Importantly, these results pointed towards a more general role for Li salts in disrupting unreactive Gilman aggregates, spawning a search for other Lipshutz‐type reagents that could offer safer alternatives to cyanide‐based preparations.
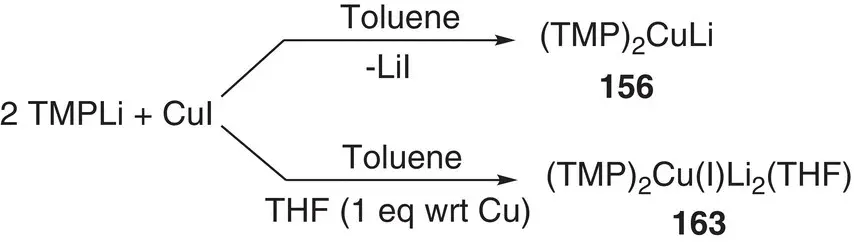
Scheme 1.35 Selective formation of Gilman and Lipshutz‐type cuprates from CuI.
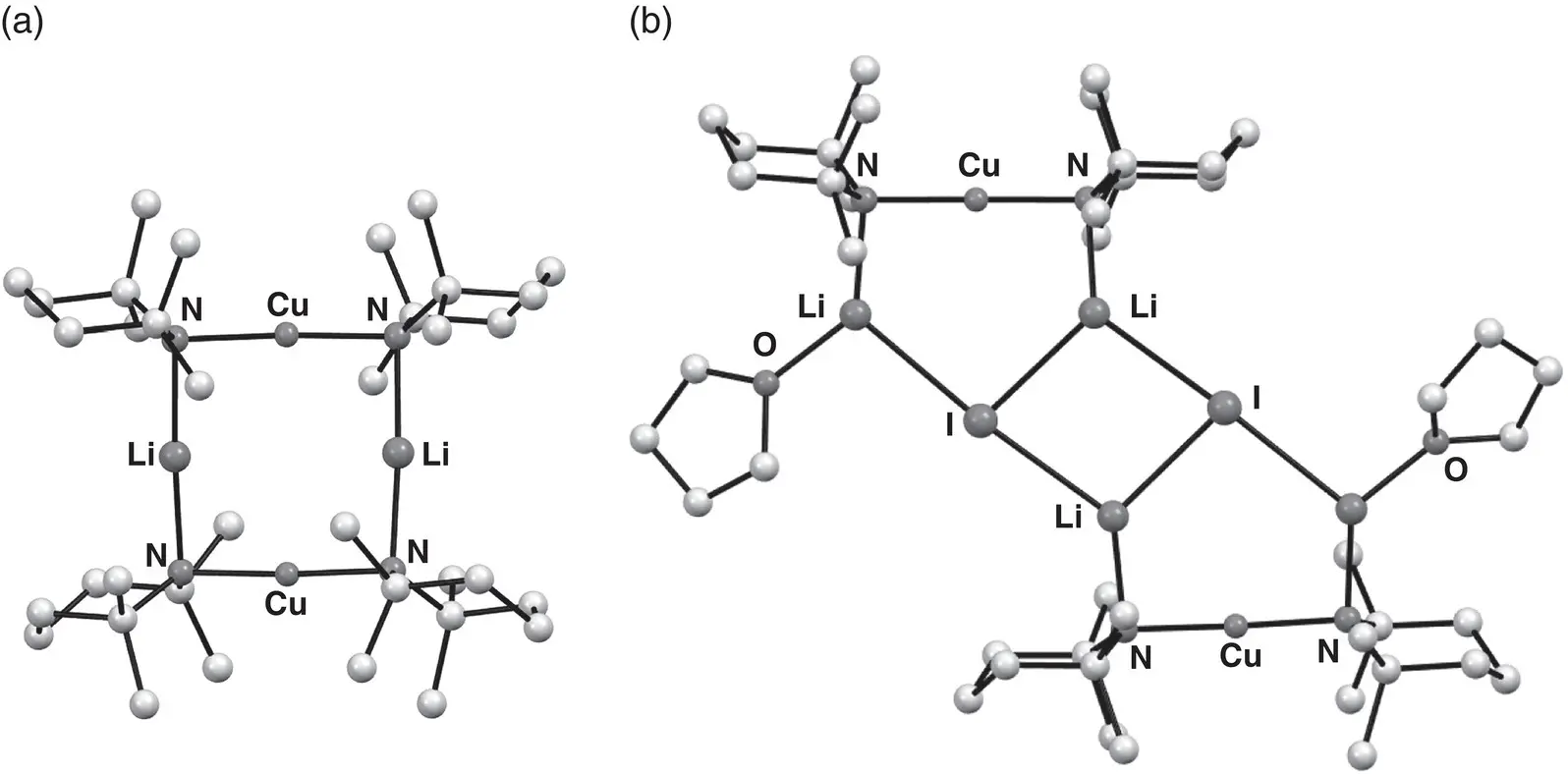
Figure 1.24 Molecular structures of (a) Gilman amidocuprate dimer of (TMP) 2CuLi 156and (b) Lipshutz‐type dimer of (TMP) 2Cu(I)Li 2(THF) 163.
Source : Adapted from Komagawa et al. [221].
Readily available copper(I) halides CuCl [222] and CuBr [223] have been investigated as sources of lithium salts for Lipshutz‐type cuprates, the structures of which have been found to be very similar to that of the dimeric iodide shown in Figure 1.24. Synthetically, in situ preparations using the putative cuprate (TMP) 2Cu(Cl)Li 2 164were found to be excellent reagents for the directed cupration of heterocycles, opening up a new route to the synthesis of pharmacologically interesting azafluorenones [222].
In an attempt to decrease the costs associated with amidocuprate preparation [224], copper(I) halides have been employed extensively in the creation of DMP‐ rather than relatively expensive TMP‐cuprates (DMP = cis ‐2,6‐dimethylpiperidide) [225]. The 2 : 1 reaction of amidolithium LDMP with CuX (X = Cl, Br, I) was therefore attempted as a route to more economical Lipshutz‐type cuprates. Remarkably, the formal replacement of two methyl groups from TMP with H‐atoms led to an entirely different structure‐type that could be viewed as an adduct of Gilman and Lipshutz‐type monomers ( Figure 1.25shows X = Br 165). In the pentametallic species seen, differences in Li–X (X = Cl, Br, I) and Li–N bond lengths were rationalized in terms of competing stabilization by hard/soft donors. Experiments in which TMP‐cuprates and DMP‐cuprates were both prepared in the presence of THF or Et 2O confirmed that the difference in structure‐types was attributable to the amido ligand rather than the Lewis base. Importantly, the inclusion of LiX (X = Cl, Br, I) in adduct cuprates was consistent with their observed reactivity in directed ortho ‐cupration. DFT calculations reinforced this view that adducts could affect ortho ‐metalation by showing that adduct cuprates represented an energetically feasible source of reactive Gilman monomers – which prior work had already suggested to represent the active species in directed ortho ‐cupration [221].
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Polar Organometallic Reagents»
Представляем Вашему вниманию похожие книги на «Polar Organometallic Reagents» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Polar Organometallic Reagents» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












