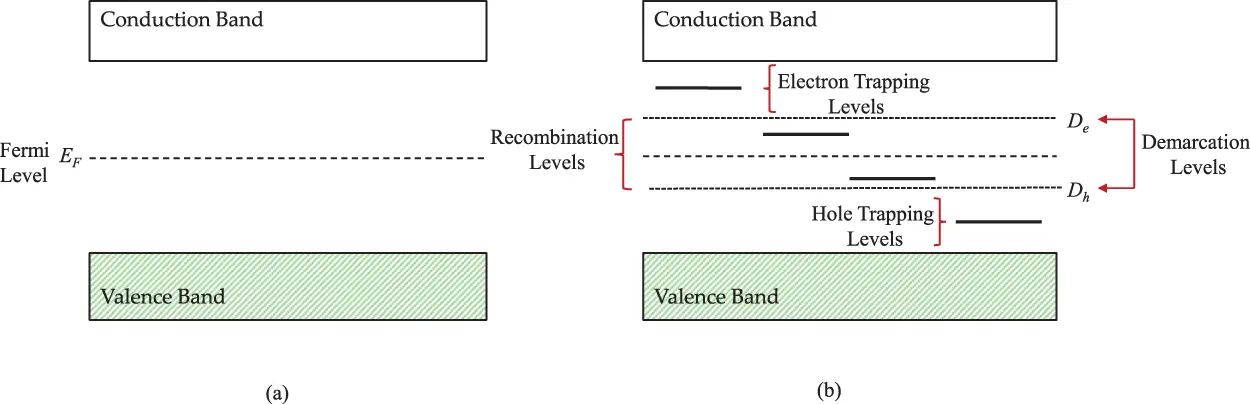
Figure 2.1 (a) Idealized energy-band diagram for a perfect crystal at equilibrium, illustrating an empty conduction band and a filled valence band. For wide-band-gap insulators, the Fermi Level ( EF ) is located mid gap. (b) A more-realistic energy-band model in which energy levels exist in the forbidden gap. Depending upon the location of the energy level (specifically, their position with respect to the band edges and the Fermi Level) the levels may be considered as “traps” or as “recombination centers,” for either electrons or holes. The virtual demarcations between them are represented by Demarcation Levels, one for electrons ( De ) and one for holes ( Dh ).
Point defects may be due to:
vacancies, where a host atom is missing;
interstitials, where a host atom occupies an off-lattice position between other host atoms;
anti-site defects, where in a host of type AB, A atoms occupy B sites, and vice-versa;
substitutional impurities, where a host atom is replaced by an impurity atom;
interstitial impurities, where an impurity atom is located in an interstitial, off-lattice position;
complex clusters of the above.
The archetypical materials for discussing defects in solids are the alkali halides, of the type A +B –. In such a material, vacancies can exist on the A or the B sub-lattice. Both A and B ions can occupy interstitial positions. Impurities can reside on either the A or the B lattice, depending on their valency, and they can also occupy interstitial sites. Depending on the valency of the impurity compared to that of the host atom, vacancies of type A or B may be required to co-exist with the impurities to maintain charge neutrality. Charge neutrality may also be maintained by the creation of Frenkel defects where vacancies of type A are charge compensated by interstitials, also of type A. Similarly, Frenkel defects involving the B sub-lattice may also occur. Alternatively, vacancies in the body of the crystal, on the A (or B) sub-lattice, may be charge compensated through the creation of equal numbers of vacancies on both the B (or A) sublattices and these are known as Schottky defects.
The local charge imbalance caused by vacancies, interstitials, and impurities may also be compensated by the localization of free charge carriers (electrons or holes) in cases where such delocalized free carriers exist. At equilibrium, there are negligible numbers of such carriers at normal temperatures (and none at zero Kelvin), but the defects can act as traps for whatever free charge carriers become available due to coulombic interactions between the free carriers and the traps. For example, trivalent rare-earth impurities (RE 3+) in an alkaline-earth halide (e.g. CaF 2) may substitute for host positive ions (anions, in this case Ca 2+). The charge imbalance results in a very strong coulombic attraction for free electrons forming divalent sites (i.e. RE 3++ e –= RE 2+). In cases where the electrons are localized at defect sites they can attain energies which are higher than the valence band energies, but smaller than the conduction band energies. Thus, the energy band diagram of a real crystal, containing these simple defect types, would be characterized by allowed energy levels in the forbidden gap, as illustrated in Figure 2.1b. The Fermi-Dirac function (Equation 1.1 in Chapter 1) demonstrates that the occupancy of energy level E , be it localized or delocalized, depends upon the temperature T and the value of E relative to the Fermi Level EF . If the band gap is such that Ec – Ev >> kT ( Ev = top of the valence band; Ec = bottom of the conduction band), then all energy levels above EF are essentially empty of electrons at equilibrium, and all those below the Fermi level are essentially full.
Point defects allow a conceptual picture of what a defect might look like in a crystal. However, these simple descriptions are far from complete. They do not include ionic polarization effects and electronic interactions with electrons and nuclei in neighboring ions. Such effects mean that “point” defects in fact exert influences on the lattice out to several lattice spacings in all directions. Consider, for example, impurity X 2+substituting for A +in ionic compound A +B –. In Figure 2.2a, a schematic ideal AB lattice is shown, typical of alkali halides. The alkali and halide sublattices are each face-centered-cubic and the picture shown in the figure is considered to stretch to infinity in all dimensions. Introduction of impurity X 2+substituting for one of the A +host ions causes the surrounding A +ions to be repelled and the B –ions to be attracted to the X 2+ion (Figure 2.2b). Such polarization effects cause a distortion to the lattice, spreading out over several atomic spacings. The polarization effects are dependent upon the dielectric constant of the medium and decrease rapidly (1/ r 2) with inter-atomic spacing ( r ). The polarization energy affects the optical and electronic properties of the material and moving electronic charges also cause subsequent changes to the polarization and displacements (Hayes and Stoneham 1985).
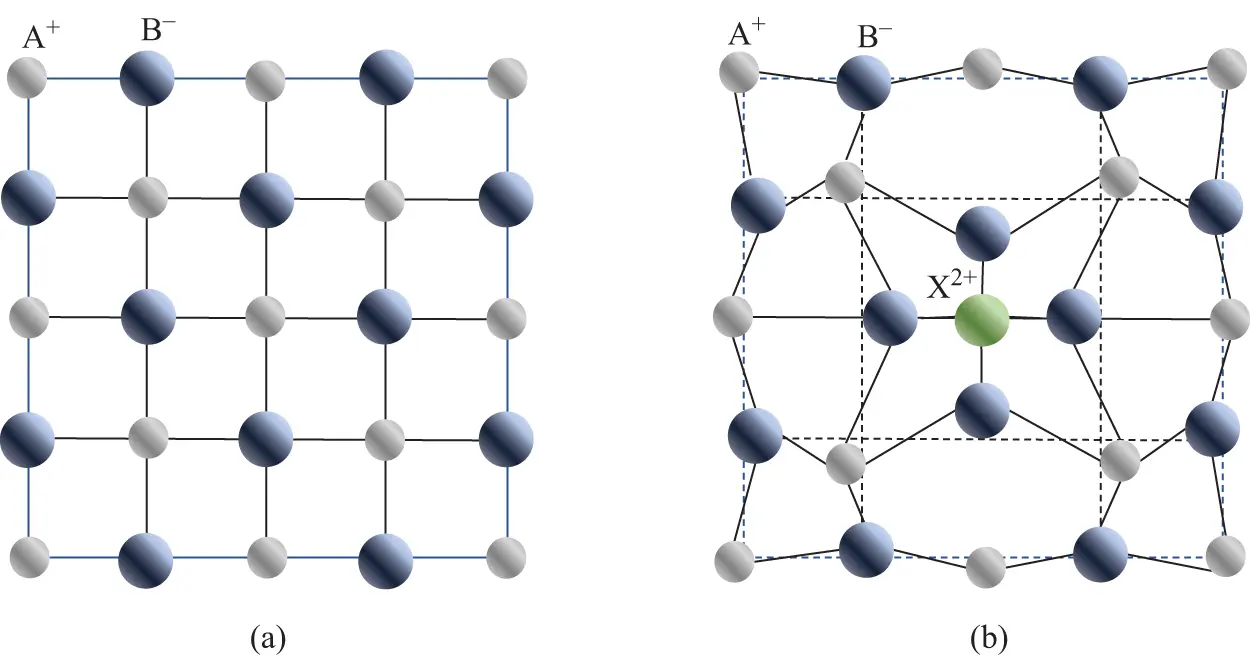
Figure 2.2 (a) An idealized lattice for an ionic crystal of the type A +B –. (b) Stylized polarization effects caused by the substitution of an A +ion with a divalent impurity ion X 2+.
The schematic illustration in Figure 2.2b is for illustrative purposes only. Modern density functional theory (DFT) calculations are able to calculate the positions of the ions when a divalent impurity is introduced into an A +B –lattice. An example is shown in Figure 2.3 for LiF doped with Mg 2+, with the Mg ion in an interstitial position. For another example, Masillon et al. (2019) reveal that when Mg 2+substitutes for Li +there is a resultant displacement of six nearest F atoms symmetrically in pairs, by 0.14 Å, 0.15 Å and 0.18 Å, while one F atom close to the Li-vacancy moves by 0.15 Å. At the same time, three neighboring Li atoms move away from their original position; two by 0.11 Å and one by 0.14 Å.
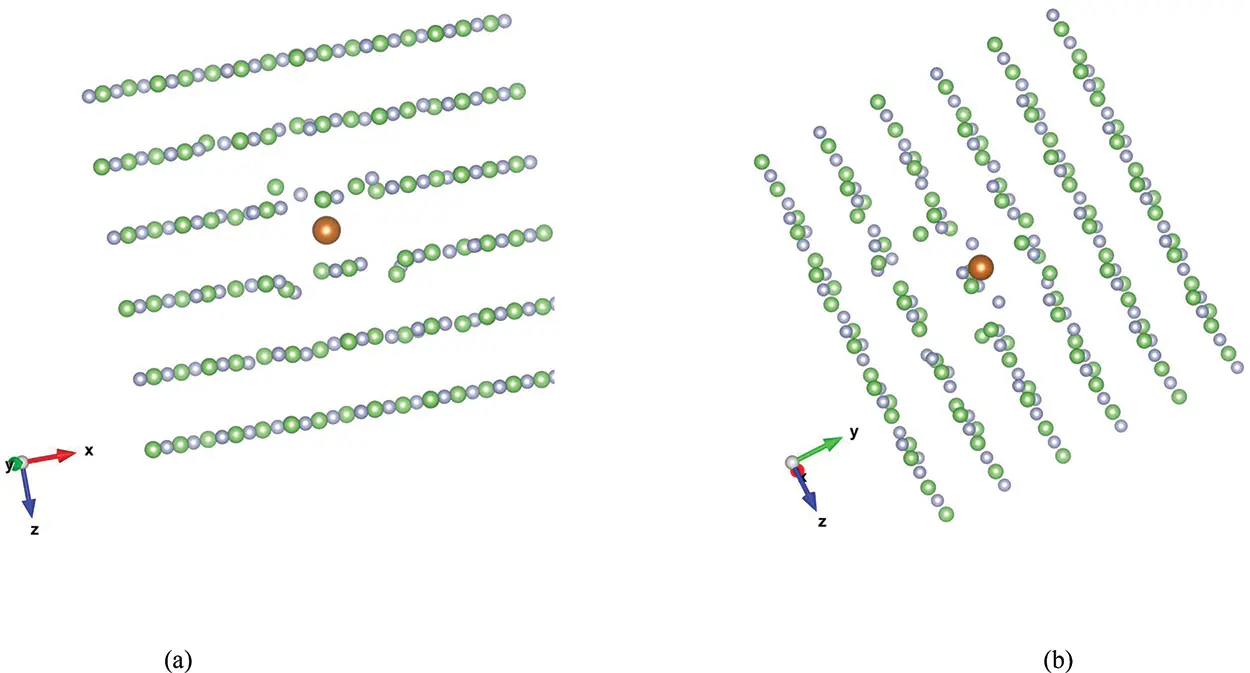
Figure 2.3 Two views, (a) and (b), of density functional theory calculations for the distortions in the lattice caused by the addition of an interstitial Mg 2+ion impurity into LiF. Li +ions are in grey; F –ions are in green, and the Mg 2+impurity ion is in orange. (Original data kindly provided by Guerda Masillon, © Guerda Masillon, UNAM, Mexico.)
The conclusion from these considerations is that a “point” defect in a lattice can exert influence over several lattice spacings and, in the certain cases, over several thousand surrounding host ions. Indeed, a “point defect” is not a “point” at all (Townsend 1992).
In a dilute system each defect can be considered “unseen” by other defects. In this definition, no matter over how many lattice spacings the defect can exert influence, there are no other defects within this sphere of influence. However, this is not always the case. For example, a divalent impurity substituting for an alkali ion can be charge compensated locally through coulombic attraction with an alkali vacancy. Using the popular TL dosimetry material LiF:Mg as an example, Mg 2+ions that substitute for host Li +ions are charge compensated by Li vacancies, which are effectively negatively charged and occupy nearest-neighbor positions along the <110> direction in the lattice. The clustering process forms dipolar complexes (Figure 2.4a), which are revealed by DFT calculations in LiF:Mg (Masillon et al. 2019). However, at room temperature this process of dipole formation does not lead to the final thermodynamic equilibrium state of the system, and further reduction in the system’s free energy can be attained by further clustering of the dipoles to form trimers, consisting of three dipoles in one of several possible configurations, an example of which is shown in Figure 2.4b (Taylor and Lilley 1982a; McKeever 1985; Gavartin et al. 1991).
Читать дальше