Poly(lactic acid)
Здесь есть возможность читать онлайн «Poly(lactic acid)» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Poly(lactic acid)
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Poly(lactic acid): краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Poly(lactic acid)»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Poly(lactic acid) Synthesis, Structures, Properties, Processing, Applications, and End of Life
Poly(lactic acid) Synthesis, Structures, Properties, Processing, Applications, and End of Life, Second Edition
Poly(lactic acid) — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Poly(lactic acid)», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
6.2.4 Crystal Structure of the β Form
As for the structure of the β form, the two types of the crystal structure were previously proposed by the X‐ray or electron diffraction data analysis: model (i) the orthorhombic type: a = 10.31 Å, b = 18.21 Å, and c (chain axis) = 9.00 Å, in which the six chains of 3/1 helical conformation are packed [17], and model (ii) the trigonal type with a = b = 10.52 Å and c (chain axis) = 8.80 Å, in which the three upward helices of 3/1 conformation are related by the space group symmetry P 3 2[18]. The present authors measured the 2D X‐ray diffraction pattern using a Mo‐Kα beam and analyzed it thoroughly ( Figure 6.2d). The several diffraction peaks intrinsic to the β form could not be indexed reasonably by using the model (ii). Besides, the observed 000 l reflections along the chain axis do not satisfy the extinction rule requested for the P 3 2space group (the appearance of 000 l reflections with l = 3, 6, 9, …) [20]. The 2D X‐ray diffraction pattern was measured again using the incident X‐ray beam of shorter wavelength (λ = 0.711 Å) than before, giving 40–50 observed diffraction spots in total [20]. The indexing of the observed peaks was made using the orthorhombic‐type unit cell:
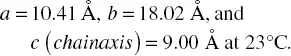
The chain takes the 3/1 helical conformation [17, 18], and the six chains are packed in the unit cell, the same as the model (i). The 18 monomeric units are contained in the unit cell. According to the International Table for Crystallography [53], the orthorhombic unit cell must contain four or eight crystallographically asymmetric units. Therefore, if the orthorhombic system is assumed, the 18 monomeric units (or six chains) cannot be divided into the asymmetric units and are difficult to correlate with each other by the symmetric relation. So, the final space group symmetry selected is P 1, which reproduced the observed X‐ray diffraction data well. However, it was impossible to determine the packing structure of many such chains uniquely because the observed diffraction peaks are relatively small in number compared with the total number of the adjustable structure parameters. As the hints to construct the crystal structure model, two important phenomena were observed:
1 The α (or δ) form transforms to the β form by the application of tensile or shear force, wherein the alternate packing structure of the upward and downward chains must be kept between them.
2 The relationships of the unit cell size among the three crystalline forms are a (α) ≈ a (δ) ≈ a (β), b (α) ≈ b (δ) ≈ b/3 (β), and c (α) ≈ c (δ) ≈ 3c (β), suggesting that the positions of the chains in the cell might not change very much before and after the structural transition from the α to β form.
Then, the chain packing structure of the α form was used as an initial model of the β form, which was enlarged three times along the b ‐axis. The thus‐constructed model consists of the alternate packing of the upward (U) chains and downward (D) 3/1 chains. The positions and relative orientations of these U and D chains were modified in various ways to obtain the best reproduction of the observed X‐ray diffraction profiles. At present, the two possible models (model 2 and model 3) are considered as the best candidates for the β form ( Figure 6.8). The U (and D) chains are surrounded by the U and D chains in a different environment depending on the local position, suggesting the frustrated structure as pointed out by Lotz et al. [18]. The comparison between the observed and calculated 1D‐WAXD profiles is presented in Figure 6.9.
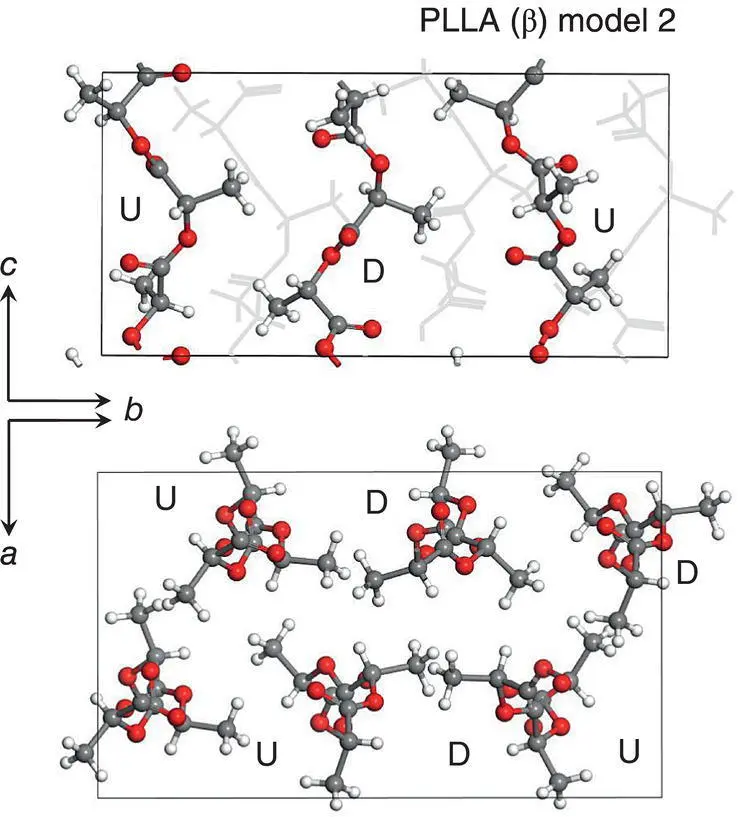
FIGURE 6.8 Crystal structure of PLLA βform (model 2).
Source: Reproduced from Wang et al., Macromolecules 2017, 50, 3285–3300
6.2.5 Structure of the Mesophase
The 2D X‐ray diffraction pattern of the uniaxially oriented mesophase, prepared by stretching the amorphous sample around T g, is shown in Figure 6.2a. The pattern is very broad and diffuse. The relative content of the mesophase increases with an increase of tensile drawing ratio of the original amorphous sample [54]. The X‐ray data analysis revealed that the oriented mesophase contains the conformationally disordered 10/3 helical chains, which are gathered together to form the small domains of about 30 Å ( c ‐axis) × 20 Å (lateral direction) size with low correlation between them [5]. By heating, the mesophase undergoes the stepwise disorder‐to‐order phase transformation to the δ and α forms [5] (refer to Section 6.3.1). These transitions were proposed to occur, not by the solid‐to‐solid process, but by the melt‐recrystallization process, although not yet confirmed [55].
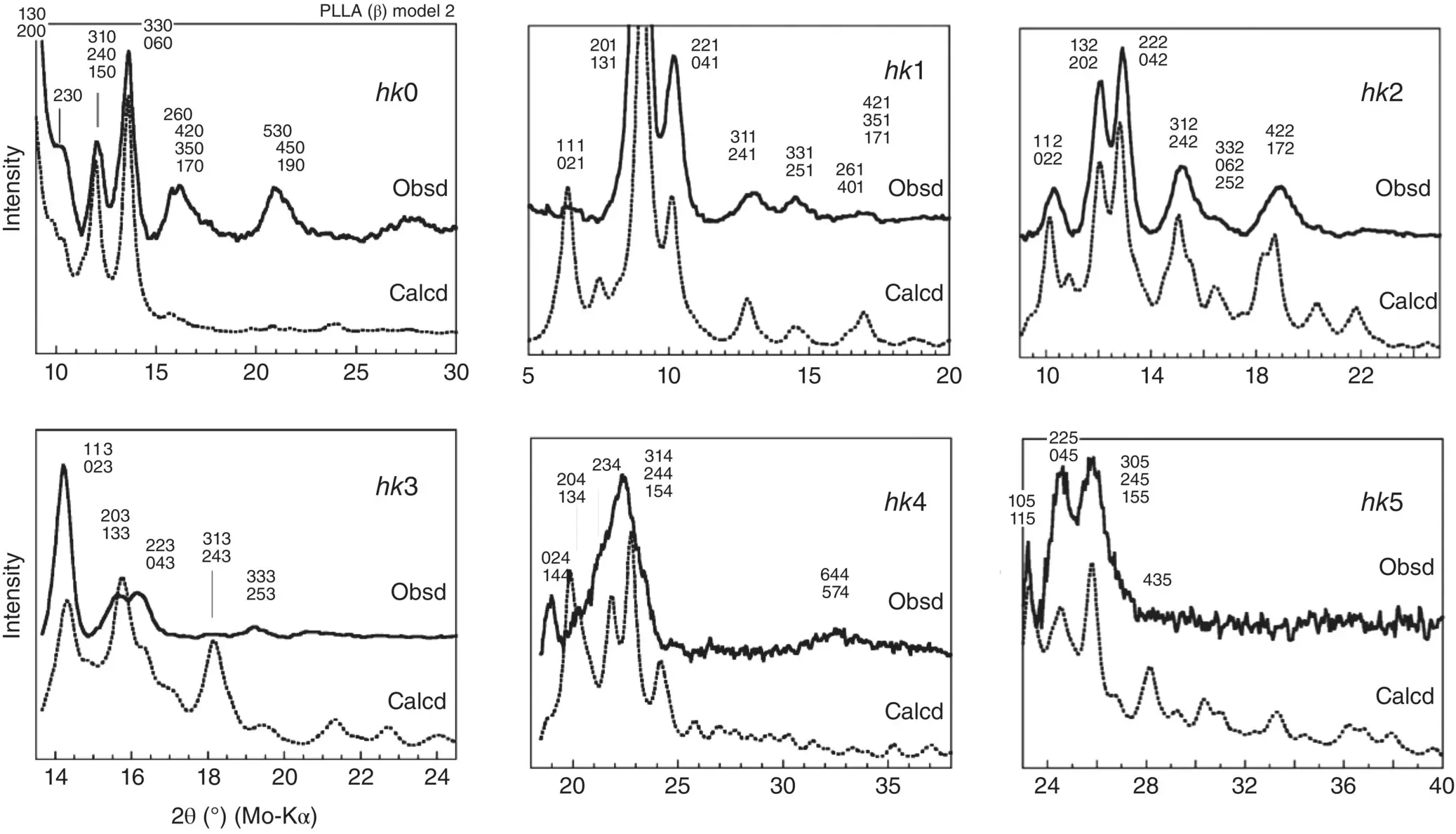
FIGURE 6.9 Comparison of the observed X‐ray diffraction profiles with those calculated for the structure model 2 of the βform.
Source: Reproduced from Wang et al., Macromolecules 2017, 50, 3285–3300.
6.3 THERMALLY INDUCED PHASE TRANSITIONS
6.3.1 Phase Transition in Cold Crystallization
The crystallization phenomenon caused by heating the amorphous sample is known as cold‐crystallization in contrast to the melt‐crystallization that occurs during the cooling process from the molten state. The temperature‐dependent 2D‐WAXD patterns were measured for the uniaxially oriented mesophase sample in the cold‐crystallization process, as shown in Figure 6.10[5]. The mesophase started to transform to the δ form at around T g.The δ form grew gradually during heating to 120°C. Once the sample was heated above 120°C, the δ form transformed to the α form. By further heating, the α form did not directly transform to the molten state, but it first changed to the mesophase once again and then to the melt. The growth of the crystallite size was estimated by analyzing the change of the half‐width of the X‐ray diffraction spots using Scherrer’s equation [52]. Figure 6.11shows the results obtained for the equatorial (200/110 peak) and meridional (0010 peak) directions. The starting mesophase has a quite small crystal size of ca . 30 Å ( c ‐axis) × 20 Å ( ab ‐plane). It changes gradually to the δ form with a larger size of ca . 75 Å ( c ‐axis) × 100 Å ( ab ‐plane). The α form developed from δ form has a larger crystal size of ca . 175 Å ( c ‐axis) × 300 Å ( ab ‐plane).
6.3.2 Phase Transition in the Melt Crystallization
As predicted from the phase diagram ( Figure 6.1), quenching the melt gives various crystalline phases depending on the quenching temperature. The X‐ray diffraction measurement revealed the details as shown elsewhere [5]. The mesophase was formed when the quenching temperature was near T g. By quenching into 100–120°C, the δ crystal was formed. Cooling to the higher temperature caused crystallization to the α form.
Figure 6.12a and b show the time dependence of the infrared absorbance at 921 cm −1band, which is common to various crystal phases, measured in the temperature jump process from the amorphous phase or from the melt [5]. In these experiments, the temperature was changed quite sharply to a preset crystallization point by using a homemade temperature jump cell [56]. The crystallization rate k was estimated from the steepest slope of the intensity‐vs‐time curves at each temperature, as seen in Figure 6.12c. The crystallization rate started to increase above 70°C and showed a maximum at around 110°C, and then decreased with increasing temperature. These curves are similar to those of the spherulite growth rate measured with an optical microscope [50, 57]. The two important points are extracted from these experimental data. One is about the crystallization of the different crystal forms: in the low temperature region (70–110°C), the δ form is produced, while the α form is crystallized in the high temperature region (>110°C), as already mentioned above. Another point is about the crystallization rate. As seen in Figure 6.12c, the cold‐crystallization gave the crystallization rate higher than the melt‐crystallization when compared at the same crystallization temperature. The higher crystallization rate in the cold crystallization phenomenon was ascribed to the higher content of the crystalline nuclei produced in the cooling process from the amorphous phase compared with that from the molten state [58, 59].
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Poly(lactic acid)»
Представляем Вашему вниманию похожие книги на «Poly(lactic acid)» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Poly(lactic acid)» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












