Poly(lactic acid)
Здесь есть возможность читать онлайн «Poly(lactic acid)» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Poly(lactic acid)
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Poly(lactic acid): краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Poly(lactic acid)»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Poly(lactic acid) Synthesis, Structures, Properties, Processing, Applications, and End of Life
Poly(lactic acid) Synthesis, Structures, Properties, Processing, Applications, and End of Life, Second Edition
Poly(lactic acid) — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Poly(lactic acid)», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
The similar situation is seen also for the crystal structures of poly(3‐hydroxybutyrate) (PHB). By adding one CH 2unit to the basic chemical formula of PLA (─[─C*H(CH 3)OCO─] n─), PHB (─[─CH 2C*H(CH 3)OCO─] n─) is obtained. One skeletal C atom is optically asymmetric and so the PHB polymer chain may take also the L and D enantiomeric species similarly to those of PLLA and PDLA. This apparently slight difference of chemical structure between PLA and PHB, however, provides the remarkably different characters with respect to the helical chain conformation and the chain packing mode, as well as the crystallization behavior itself. Additionally, there are many kinds of aliphatic polyesters, which can show relatively high biodegradability comparable with that of PLA and PHB. For example, poly(ethylene adipate) (PEA) has the linear chemical formula without CH 3side groups, ─[─OCH 2CH 2OCO(CH 2) 4CO─] n─. In some cases, PEA is blended with PLA or PHB to control the biodegradability. To understand the crystallization behavior of PEA and PLA in these blends, it is indispensable to know the structure of PEA at the starting point.
In the present chapter, the crystal structures and phase transition behaviors of PLA are mainly discussed, but, at the same time, such related polyesters as PHB and PEA are also reviewed to extract the similarity and differences between these polyester compounds, from the structural points of view.
Although many review articles have been published about the structures, the phase transition behaviors and the relation between structure and property of these polymers [38–41], we need to realize the most reliable and accurate information, which were derived mainly from the quantitative analyses of the X‐ray diffraction and vibrational spectroscopic data, are introduced in the present chapter.
6.2 STRUCTURAL STUDY OF PLA
6.2.1 Preparation of Crystal Modifications of PLA
To elucidate the detailed crystal structures and phase transition behaviors of PLLA, X‐ray diffraction method of highly oriented and highly crystalline samples is most useful. Figure 6.1shows that the various crystal modifications are obtained depending on the sample preparation conditions. When the sample in the molten state is quenched into ice water, predominantly amorphous‐phase PLA is obtained [4, 5]. Casting PLA films using chloroform solution at room temperature also produces amorphous phase. The oriented PLLA sample of the mesophase is prepared by stretching the melt‐quenched sample by four to five times the original length near the glass transition temperature ( T g~ 60°C) [5]. The oriented δ form is obtained by annealing the as‐drawn mesophase in the temperature range of 70–120°C. Annealing of the δ form at a higher temperature of 120–170°C induces a phase transition to the α form [5, 7, 9, 14]. The oriented β form cannot be obtained easily by the usual heat treatment of other such crystal modifications as the δ and α forms. To prepare the highly oriented pure β form, a high shear or tensile stress has to be applied to the oriented α form at a temperature higher than 120°C [17–20,42–45]. The γ form is obtained as a single crystal by casting from hexamethylbenzene solution [21, 22]. A PLLA–CO 2complex is prepared by treating the PLLA sample with supercritical fluid CO 2. The desorption of CO 2under vacuum at room temperature gives the empty α″ form. PLLA forms a crystalline complex with organic solvents like cyclopentanone (CPO) and N , N ‐dimethylformamide (DMF), which is known as the ε form [25]. This complex is stable only at a low temperature; it transforms spontaneously to the α (or δ) form by leaving the sample at room temperature.
PLLA and PDLA are enantiomers with the same chemical formula but with the opposite configuration around the asymmetric carbon atoms or the opposite optical activity. The blend sample of PLLA and PDLA at 1 : 1 molar ratio was found to form the so‐called stereocomplex (SC) [27]. However, the SC sample can be obtained in a wider range of PLLA/PDLA ratio of 7/3–3/7 [37, 46, 47].
Figure 6.2shows the typical WAXD patterns of the uniaxially oriented PLA samples of the mesophase, δ, α, and β forms [5, 9, 14, 20], where an incident X‐ray beam is a graphite‐monochromatized Mo‐Kα line (wavelength λ = 0.711 Å). Compared with the X‐ray diffraction patterns observed for the general crystalline polymer samples, the α crystal form shows the anomalously beautiful X‐ray diffraction pattern with many sharp spots, reflecting the well‐developed crystal domains with highly regular chain packing mode. The diffraction pattern of the δ form is similar to that of the α form but diffuse as a whole, and the several characteristic diffraction peaks of the α form are lack, indicating that the δ form is not simply a disordered α form, but it is a crystalline form independent of the α form. The mesophase shows the further poor and diffuse diffraction pattern with the similar characteristic structural feature to those of the α and δ forms. The β form shows the remarkably different diffraction pattern from those of the abovementioned α and δ forms and meso phase. The streaks are more remarkable in the β form.
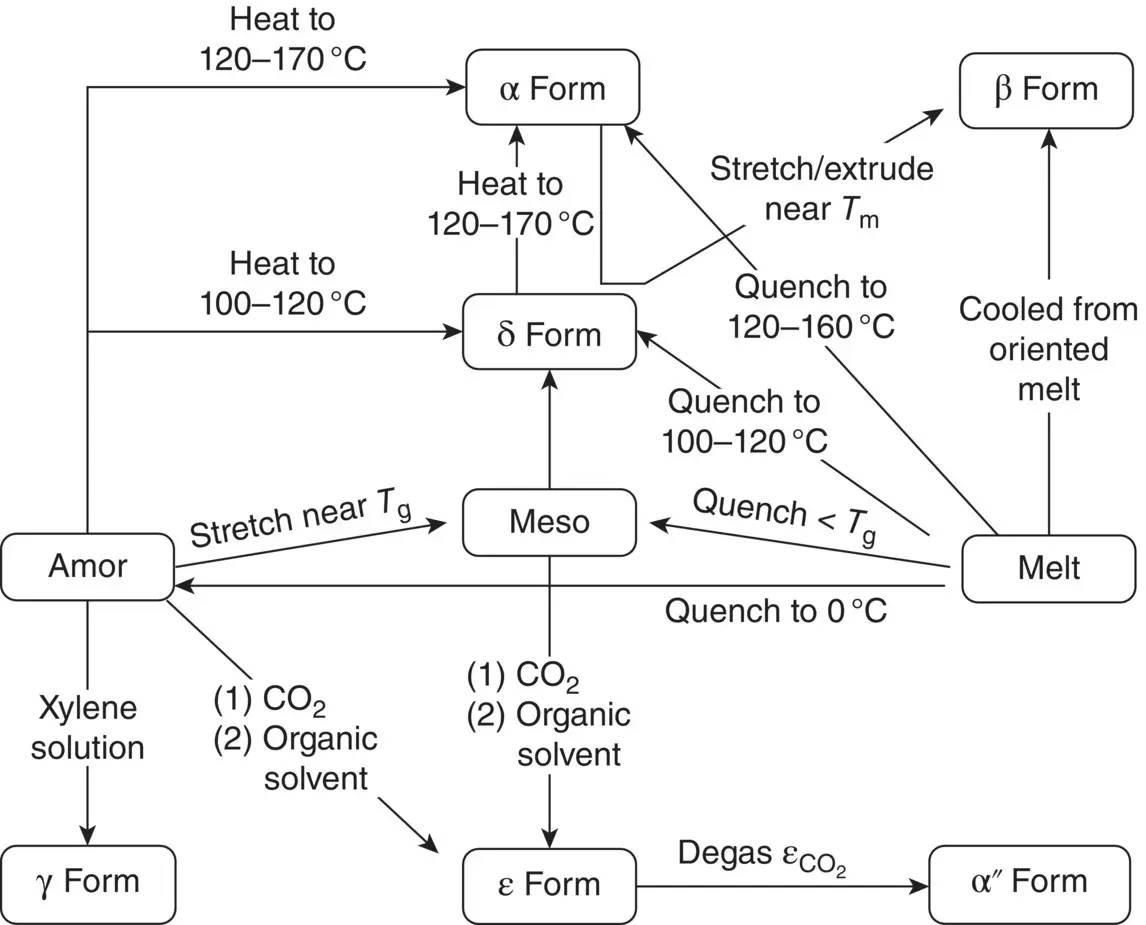
FIGURE 6.1 Sample preparations and phase transitions of the various crystal modifications of PLLA.
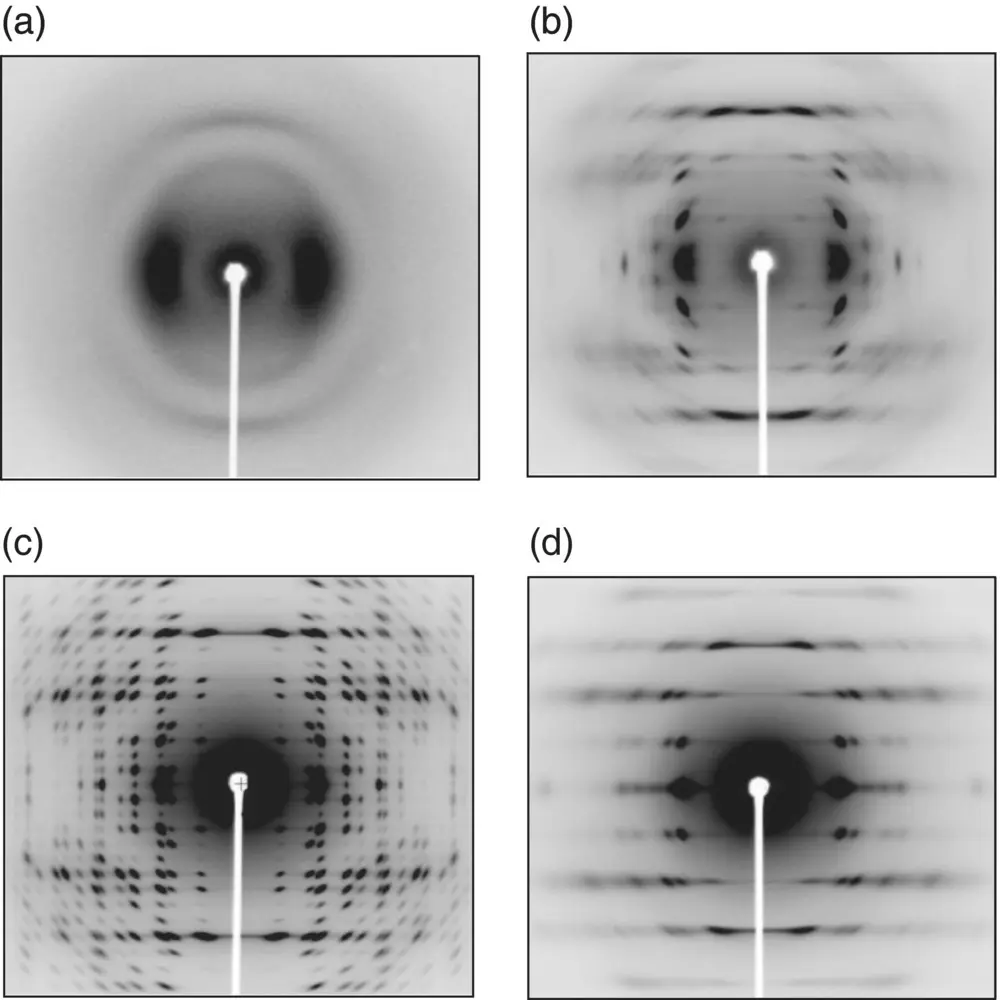
FIGURE 6.2 2D‐WAXD patterns of PLLA (a) mesophase, (b) δform, (c) αform, and (d) βform.
Source: (a)–(c): Reproduced from Wasanasuk et al., Macromolecules 2011, 44, 9650–9660; (d): Wang et al., Macromolecules 2017, 50, 3285–3300.
6.2.2 Crystal Structure of the α Form
The crystal structure of PLLA α form proposed at first was of the orthorhombic unit cell of a = 10.7 Å, b = 6.45 Å, c (chain axis) = 27.8 Å [15], which contains the two chains of 10/3 helical conformation [13, 48], where 10/3 indicates that the 10 monomeric units are contained and the 3 turns in the repeating period. The internal rotation angles of the skeletal chain are approximately expressed as the repetition of TTG where T and G are trans and gauche bonds, respectively. The crystal structure was refined by Sasaki et al. by assuming the space group symmetry P 2 12 12 1[13]. However, several unsolved problems are still relevant in the abovementioned crystal structure analysis of the α form. For example, if the structure of the P 2 12 12 1space group is assumed correct, the molecular chain must be deformed more or less from the regular and uniform 10/3 helical conformation, since the unit cell possesses only the 2 1screw symmetries. If the 2 1screw axis passes through the center of the molecular chain along the c axis, the five monomeric units should form one crystallographically asymmetric unit. The total number of the atomic coordinates to be determined is remarkably large, making the structure analysis greatly difficult. A more serious problem is the usage of the space group P 2 12 12 1itself.
The observed X‐ray diffraction pattern shows a series of 00 l reflections containing the odd l values (003, 007 etc.) in addition to the even 00 l peaks. This is not consistent with the extinction rule (00 l with even l values) required for the space group P 2 12 12 1.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Poly(lactic acid)»
Представляем Вашему вниманию похожие книги на «Poly(lactic acid)» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Poly(lactic acid)» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












