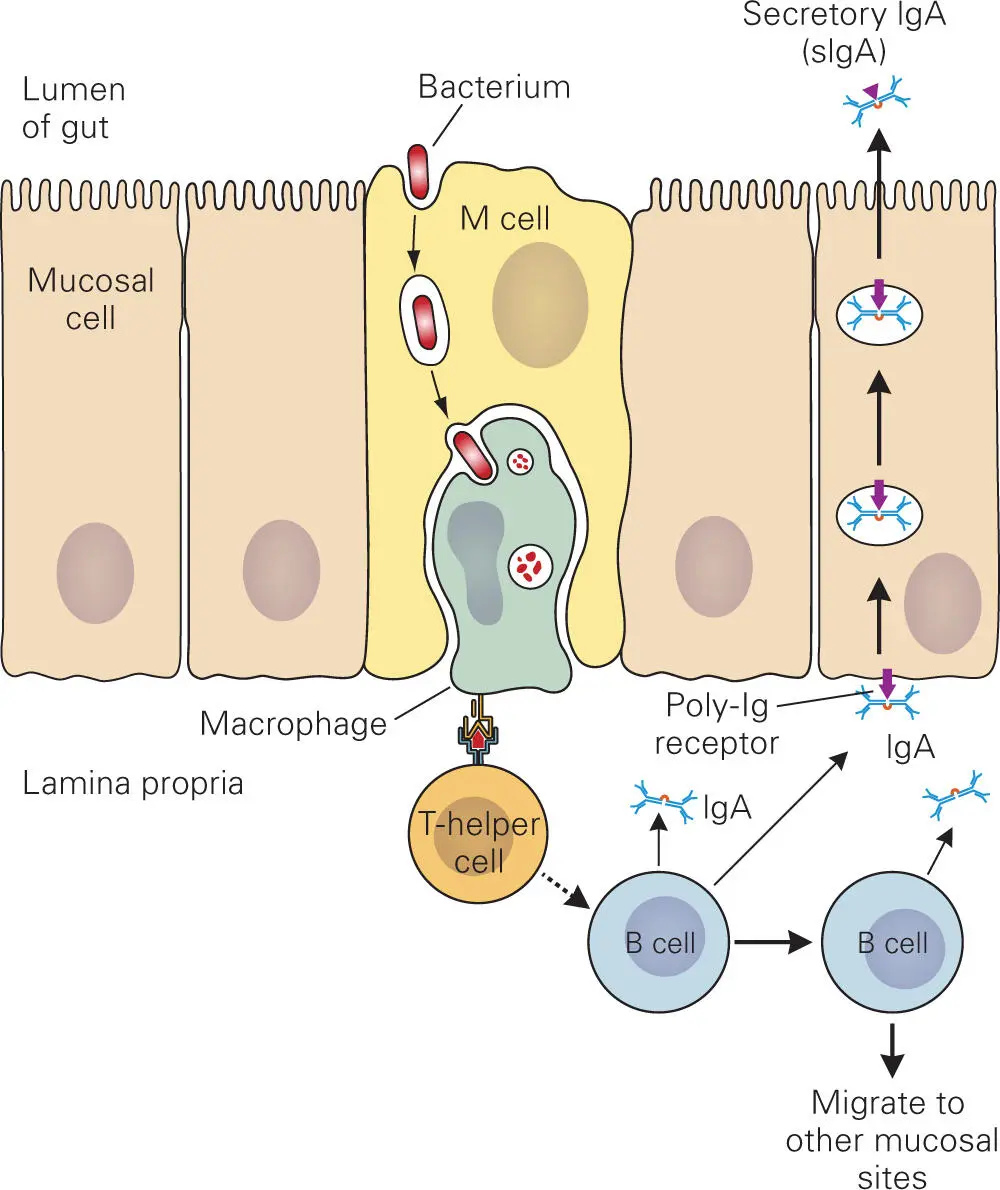
Figure 4-12. Cells of the GALT that confer mucosal immunity. M cells and their associated macrophages and lymphoid cells (T and B cells) are sometimes called follicles. Collections of such follicles in the gut are called Peyer’s patches. M cells sample the contents of the gut lumen and transfer the antigens to closely associated resident macrophages, which in turn ingest the bacteria and present antigens to the underlying T cells that then stimulate nearby B cells to produce IgA. The IgA binds to receptors on the basal surface of the mucosal epithelial cell and is transcytosed across the cell and secreted into the lumen of the gut as sIgA.
When the GALT is stimulated, one outcome is production of IgA (see Figure 4-12). Dimeric IgA is produced by plasma cells in the lamina propria at mucosal sites. The secretory piece is acquired when IgA dimer is transported through the mucosal epithelial cell into mucosal secretions covering various mucosal surfaces. IgA dimer binds to the poly-Ig receptor on the basal surface of mucosal cells and is then taken up by endocytosis and transported in vesicles, through a process called transcytosis, to the apical surface, where it is released into the lumen of the gut. Release involves proteolytic cleavage of the poly-Ig receptor, where the secretory piece comes from the portion of the receptor that remains attached to the IgA, making it sIgA. sIgA can trap microbes in mucus because the Fc portion of sIgA binds to glycoprotein constituents of the mucin and the Fab portion binds to antigens on the microbes in the gut. By trapping the microbes in the mucus layer, the sIgA-antigen-mucin complex essentially forms a protective barrier that blocks the microbes and their toxic products from gaining access to the epithelial cell layer. Mucus laden with sIgA-coated microbes is then sloughed off and excreted from the body.
The mucosal and skin tissues that have contact with the external environment are also constantly patrolled by DCs (APCs), which engulf and kill the invading microbes and process the antigens for presentation to the adaptive immune system. Once activated, DCs migrate to the lymph nodes, where they present the antigens to Th cells and stimulate adaptive immunity. The Langerhans cells of the epidermis are the DCs of SALT.
As part of the mucosal immune system, some T cells and B cells stimulated by antigen processing at the GALT can migrate to other mucosal sites and vice versa. Thus, stimulation at one of the MALT sites can transfer to other sites, resulting in general mucosal immunity. The first evidence for this feature of the mucosal immune system came from elegant experiments performed by Husband and Gowans in the late 1970s. These researchers excised a segment of the small intestine from a rat, preserving its vascular and lymphatic supplies, and reconnected the ends of the intestinal segment to the skin surface of the animal, forming a so-called Thiry-Vella loop ( Figure 4-13). They then introduced an antigen, in this case cholera toxin (CT), into the loop and found that sIgA was secreted in not only the immunized loop segment, but also in a second such loop (when made) and in the main intestine. Their results demonstrated that introduction of an immunogen at one site could confer mucosal immunity at a remote site. This characteristic of the MALT system is what makes oral vaccines feasible. Initially, oral vaccines stimulate the GALT, but sIgA against vaccine antigens is later detectable in other MALT sites. Thus, an oral vaccine can also be used to elicit immunity to respiratory and, presumably, urogenital pathogens.
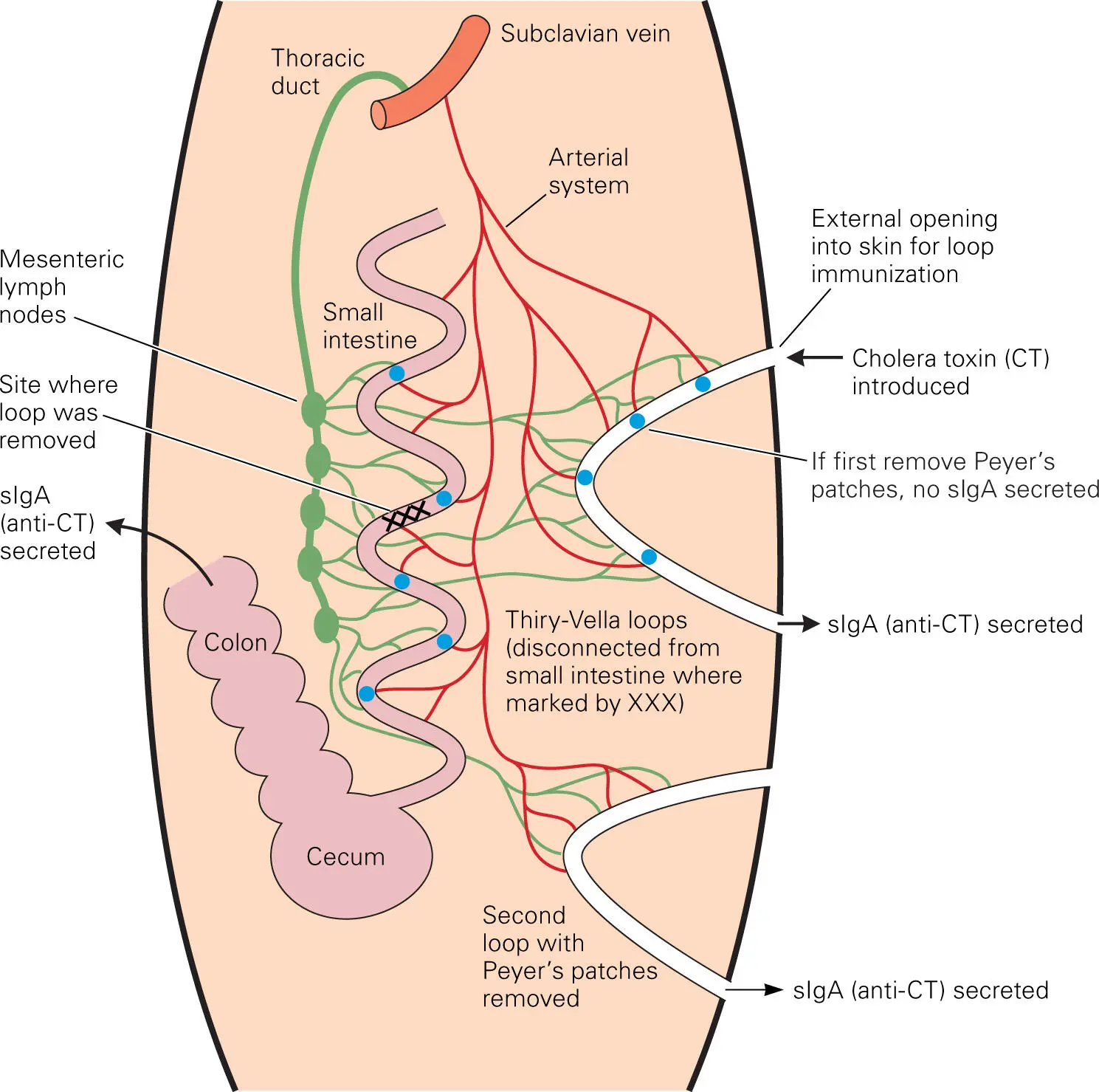
Figure 4-13. Classic experiment by Husband and Gowans demonstrating mucosal immunity at remote sites. The experimental setup involved isolating Thiry-Vella loops of the small intestine of rats and connecting them to the skin, preserving the associated vascular and lymphatic systems attached to the loops. The IgA immune response to administering immunogen (cholera toxin) to the main intestine through the oral route or through the skin opening of the loop could then be observed. Results showed that local immunization through the isolated loops that contained Peyer’s patches generated Th cells and B cells that circulated through the lymph and blood to populate other mucosal sites. Based on Husband AJ, Gowans JL. 1978. J Exp Med 148:1146–1160.
Currently, efforts are being made to develop vaccines administered by inhalation, so that stimulation of the nasal MALT (NALT) would produce an sIgA response at other MALT sites. These vaccines would have the advantage of not having to pass through the stomach. Developing vaccines that target the GALT means developing vaccines capable of surviving the low-pH/protease-rich stomach environment, a barrier that has proven problematic in many cases. Administering vaccines by rectal or vaginal suppositories is theoretically possible, but this strategy has not been actively pursued to date. On the other hand, the SALT is gaining in attraction as a target for vaccine development (more on vaccines in chapter 17).
Activation of the GALT can also lead to production of cytotoxic T cells. These cells probably remain on the basal side of the mucosa, although it is possible that during an infection some of them migrate to the apical surface, especially in areas where damage to the mucosa has occurred. GALT cytotoxic T cells are important for protection against viral infections of the GI tract and some bacterial infections in which the bacteria multiply inside mucosal cells.
One of the many mysteries swirling around the intestinal immune system is the role of a particular type of mucosal cell called γδ T cells. The majority of γδ T cells are CD8+ T cells and would thus be grouped with CTLs. However, whereas CTLs have TCRs composed of αβ chains ( Figure 4-8), the intestinal epithelial lymphocytes (IELs) have T-cell receptors composed of related but somewhat different γδ chains. These γδ T cells account for less than 4% of circulating CD8+ T cells, but they account for as much as 10 to 15% of the mucosal T cells found in the GI tract. In some regions, such as the colon, the levels may be as high as 40%.
Unlike αβ T cells, γδ T cells seem to recognize only a limited number of cell-surface antigens. Also, γδ T cells seem to bypass antigen presentation by MHCI and MHCII on the surfaces of macrophages and DCs and directly recognize nonpeptide antigens that have not been processed. Indeed, they are believed to play a predominant role in lipid-antigen recognition via CD1 complexes ( Figure 4-6C). γδ T cells also respond to two human protein complexes related to MHC I, MICA and MICB. These proteins are displayed on the surface of cells that are stressed (e.g., by being infected) and are recognized by NKG2D receptors present on γδ T cells, as well as NK cells, which results in activation of cytotoxic responses that kill the stressed cells. γδ T cells also produce cytokines that stimulate αβ CD8+ cytotoxic T cells to migrate to the area and eliminate infected cells.
As with other immune responses, the GALT has a downside. Normally, bacteria and other viable antigens that pass through M cells are killed by the resident GALT macrophages. Some bacterial pathogens, however, have acquired the ability to avoid this fate and exploit the GALT as an entryway into the body. Because the M cell is an antigen-sampling cell, it usually does not take up substantial amounts of an antigen because only a few bacteria or other antigens are sufficient to stimulate a mucosal immune response. The bacteria that use the GALT as an entryway into the body, such as Salmonella enterica or Yersinia pseudotuberculosis, invade M cells or use them to move across the epithelial layer (see Figure 4-14).
Читать дальше














