TABLE 1.14 Examples of viral diseases
| Family and virus |
Disease(s) |
| Parvoviridae (DNA, nonenveloped) |
| Human parvovirus B19 |
Erythema infectiosum (fifth disease) |
| Minute virus of mice |
Cell line contamination, oncolysis |
| Papovaviridae (DNA, nonenveloped) |
| Human papillomavirus |
Cervical cancer, genital warts |
| Picornaviridae (RNA, nonenveloped) |
| Poliovirus |
Poliomyelitis |
| Rhinoviruses |
Common cold |
| Coxsackievirus A16 |
Foot-and-mouth disease |
| Retroviridae (RNA, enveloped) |
| HIV type 1 |
AIDS |
| Human T-cell leukemia virus type 1 |
Human T-cell leukemia |
| Orthomyxoviridae (RNA, enveloped) |
| Influenza viruses A, B, and C |
Influenza, pharyngitis |
| Hepadnaviridae (DNA, enveloped) |
| Hepatitis B virus |
Hepatitis |
| Poxviridae (DNA, enveloped) |
| Variola virus |
Smallpox |
| Vaccinia virus |
Smallpox vaccine |
| Rhabdoviridae (RNA, enveloped) |
| Rabies virus |
Rabies, paralysis |
| Vesicular stomatitis virus |
Similar to foot-and-mouth disease; flu-like |
| Coronaviridae (RNA, enveloped) |
| Human coronavirus |
Severe acute respiratory syndrome, colds |
| Mouse hepatitis virus |
Wasting syndrome |
| Herpesviridae (DNA, enveloped) |
| Herpesvirus (herpes simplex virus types 1 and 2) |
Conjunctivitis, gingivostomatitis, genital herpes, meningitis |
| Varicella-zoster virus |
Chickenpox/shingles |
Bacteriophages (commonly known as phages) are mostly DNA viruses (e.g., the T3, T7, and lambda [λ] phages are E. coli viruses), although some RNA viruses have been described (e.g., nonenveloped MS2 and enveloped ϕ6 E. coli phages). Bacteriophages have been studied for many years as genetic-engineering tools, but they have other practical applications, including uses in typing of bacteria and as indicators of fecal contamination in water and limited medical applications (such as antibacterials). Lactobacillus phages are a significant contamination concern in the dairy industry. Phages are considered to be resistant to biocides, like other animal and plant viruses, and are therefore used to investigate biocidal activities (e.g., MS2 phage) and modes of action. They can be routinely cultured and purified in most bacteriology laboratories.
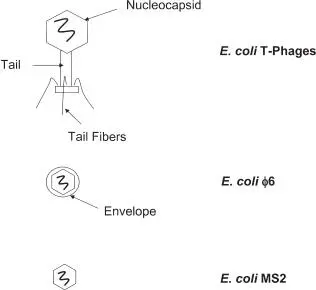
FIGURE 1.12 E. coli bacteriophages. The T-phages are complex DNA viruses; MS2 and ϕ6 are RNA viruses, with ϕ6 enveloped.
Two other groups of infectious agents are also considered “viruses” but have unique morphologies. The first are viroids, which are devoid of protein and appear to consist of naked RNA molecules. The second are proposed to be devoid of a nucleic acid and are termed “prions”; these are discussed in further detail in section 1.3.6. Viroids are known to infect only higher plants and have been identified as the causes of a number of crop diseases. Examples are potato spindle tuber viroid, coconut cadang-cadang viroid, and tomato apical stunt viroid. They consist only of small, circular RNA sequences that range in size from 246 to 375 nucleotides. It is interesting that their sequences do not encode proteins and that they are dependent on the host for replication in the cell nucleus. Although at first it would seem that these agents would not survive well in the environment, their structures are somewhat protected by forming double-stranded portions (by base pairing) within their circular, single-stranded structures. Although no human viroids have been identified, hepatitis D (delta) virus is similar to a viroid and is known as a satellite virus. A satellite virus is an agent that consists of a nucleic acid and that depends on the coinfection of a host with another virus, which is required for its replication. Hepatitis delta virus appears to be a defective transmissible pathogen that is dependent on hepatitis B virus. It consists of a circular RNA molecule (~1,680 bp), but unlike a true viroid, it does encode a capsid protein. The virus consists of a nucleocapsid of 60 proteins surrounding the RNA molecule and an external envelope of lipid and hepatitis B surface antigens.
Prions are unique infectious agents that are composed exclusively of protein and do not appear to have an associated nucleic acid. The protein in question is a normal cellular protein (cellular prion protein [PrP c]) that is expressed in many body tissues and in all vertebrates, including humans and animals. The proteins are produced in cells as long chains of amino acids (known as the primary structure), which then fold to make structural and functional (e.g., enzyme) forms (e.g., secondary and tertiary structures). The exact function of the PrP protein is unknown, but it is known to be a eukaryotic cell membrane-associated glycoprotein. Like other cellular proteins, PrP is manufactured by the cell and can be subsequently broken down by normal cellular processes ( Fig. 1.13).
However, PrP appears to be able to change its conformational secondary structure into insoluble, “infectious” forms (PrP Sc). The conformational change to PrP Screnders the protein highly resistant to normal cellular degradative processes, leading to accumulation and cell damage or death, with particular consequences to neural tissues. More specifically, an insoluble portion of the protein (PrP 27-30, a 27- to 30-kDa protein) accumulates to form amyloid deposits in the brain. Therefore, the protein primary structure does not change, but the protein secondary structure is radically altered to give an overall greater proportion of β-sheets over α-helices in the folded protein structure ( Fig. 1.14). What triggers this reaction is currently unknown; PrP Scitself has been shown to be involved in the transition, but it may also require other, yet unidentified factors.
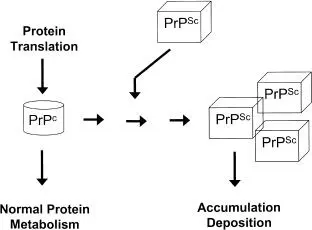
FIGURE 1.13 Theory of prions as infectious proteins. PrP cis the normal form of the protein, and PrP Scthe abnormal form.
Prions are the causative agents in a group of diseases known as transmissible spongiform encephalopathies. Animal (scrapie in sheep and bovine spongiform encephalopathy in cattle) and human (classical Creutzfeldt-Jakob disease [CJD] and variant CJD) diseases have been shown to be infectious prion diseases. Some forms have also been found to be inherited, e.g., familial CJD is responsible for ~10% of CJD cases and Gerstmann-Sträussler-Scheinker syndrome, due to modifications in the PrP-encoding gene. Human diseases are considered very rare; for example, CJD is the most common human transmissible spongiform encephalopathy, with an approximate rate of 1 to 1.5 cases per 1,000,000 population. Animal diseases are considered more widespread; for example, scrapie is estimated to affect 4 to 8% of sheep. Prions have been shown to be transferred in contaminated tissues (including infected foods, neural tissues, and blood) and on the surfaces of contaminated instruments (surgical devices). Zoonotic transmission to humans has been reported, with bovine spongiform encephalopathy now widely accepted as the source of variant CJD in humans. Finally, some researchers have speculated that other diseases that are associated with the deposition of protein (for example, the neurodegenerative diseases Parkinson’s and Alzheimer’s diseases) could also be linked to infectious agents; these reports, however, remain to be substantiated.
Читать дальше














