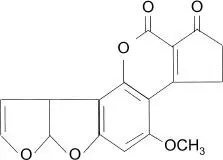
FIGURE 1.16 Typical fungal aflatoxin structure.
Mycotoxins also have multiple effects on target cells, including membrane damage, cell death, and free-radical damage. Aflatoxins have been particularly associated with food and feed (grain) contamination and have been shown to be carcinogenic. Some fungal cell wall components (like β-1,3-glucan) are also considered toxins and can cause allergic reactions, including coughing and other respiratory effects.
Many algae also produce toxins, which are often associated with contaminated water. These include hepatoxins (in particular, from blue-green algae), neurotoxins, cytotoxins, and endotoxins (similar to gram-negative bacteria, the LPS from the outer membrane of the algal cell wall).
1.4 GENERAL CONSIDERATIONS
1.4.1 Microbial Resistance
Different types of microorganisms vary in their responses to antiseptics, disinfectants, and sterilants. This is hardly surprising, in view of their different cellular structures, compositions, and physiologies (see section 1.3). Traditionally, microbial susceptibilities to biocides have been classified based on these differences ( Fig. 1.17). Bacterial spores are generally considered the organisms most resistant to antiseptics, disinfectants, and sterilants, although prions have shown marked resistance to many physical and chemical processes (see section 8.9). It is important to note that this classification is considered only a general guide to antimicrobial activity and can vary depending on the biocide, formulation, or process under consideration. For example, while the profile shown in Fig. 1.17may be considered applicable to heat-based processes, some fungal spores can demonstrate greater resistance to non-ionizing-radiation methods, and some protozoan oocysts are relatively sensitive to heat but resistant to chemical sporicides. Some extremophiles can also show atypical patterns of resistance to various biocides (see section 8.3.10). The resistance of microorganisms to biocides is considered in more detail in chapter 8.
In addition, it is clear that the resistance of a microorganism also depends on direct contact with the biocide and is affected by many other associated variables, including the following:
The actual microbial strain and culture conditions for growth
The growth phase of the microbial culture (population and exponential-versus stationary-phase growth)
The type of associated surface and/or medium (water, plastic, metals, paper, etc.)
The presence of organic and/or inorganic soils
Presence within its normal environmental conditions, e.g., within a biofilm (see section 8.3.8)
A range of microorganisms can be chosen to establish the broad-spectrum activity of a product or process, depending on the required or desired application. For example, a sterilizing agent is expected to be effective against viruses, fungi, protozoa, mycobacteria, and other bacteria, including bacterial spores. Bacteria (including spores), fungi, mycobacteria, and to a lesser extent viruses are most commonly used as test microorganisms. Considering the multitude of microorganisms and applications, it is common to use surrogates as test organisms to establish the broad-spectrum efficacy of a product or its antimicrobial activity against a class of microorganisms ( Table 1.17). Despite acceptance of these surrogates, in some cases the specific test organisms are used, or are required to be used, to verify the claimed antimicrobial activity.
1.4.2 Evaluation of Efficacy
The antimicrobial activity of a biocide or a biocidal process can be investigated using a variety of methods that can range from simple laboratory tests to evaluation under actual use conditions. These tests not only are important in the investigation of biocides and the development of products and processes but are also the basis for the regulatory clearance, labeling, and use of antiseptics, disinfectants, and sterilization processes. The various tests and requirements can vary considerably, and there are currently no standardized requirements that apply to all situations. Most countries specify particular test methods to verify antimicrobial activity, but these also vary for the particular application (e.g., medical, dental, agricultural, water disinfection, or industrial), type of biocide or process, and use of the formulation or process (e.g., preservation, antisepsis, disinfection, or sterilization).
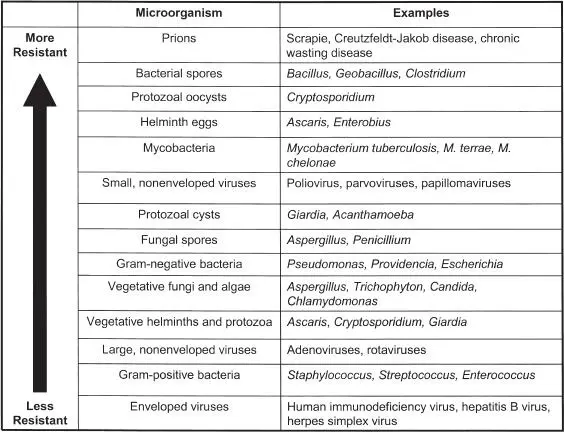
FIGURE 1.17 General microbial resistance to biocides and biocidal processes. This classification can vary depending on the biocide or biocidal process under consideration.
TABLE 1.17 Examples of surrogate microorganisms used to test and verify antimicrobial activities of biocides, products, and processes
| Efficacy claim |
Surrogate |
Example(s) of use |
| Sporicidal |
Bacillus atrophaeus, Bacillus cereus, Clostridium sporogenes |
General disinfectant and sterilant testing; sterility assurance testing for sterilization processes |
| Fungicidal |
Trichophyton mentagrophytes, Aspergillus niger, Candida albicans |
General disinfectant testing |
| Bactericidal |
Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella enterica serovar Choleraesuis, Enterococcus hirae, Escherichia coli, Serratia marcescens |
General antiseptic and disinfectant testing |
| Virucidal |
Poliovirus, adenovirus, herpesvirus, bacteriophages (e.g., Lactococcus phage F7/2) |
General antiseptic and disinfectant testing |
| Mycobactericidal |
Mycobacterium bovis, Mycobacterium terrae, Mycobacterium smegmatis |
General disinfectant testing |
| Oocysticidal |
Cryptosporidium parvum |
General disinfectant testing |
1.4.2.1 SUSPENSION TESTING
Suspension tests are widely used under laboratory conditions in the development, verification, and registration of biocidal products. The simplest test is the determination of the MIC—the lowest concentration of a biocide that inhibits the growth of a test organism—which is widely used in the evaluation of antibiotic activity against bacteria. A series of biocide dilutions (usually in growth media specific for the test organism) are inoculated with a known concentration of the test organism and then incubated to determine the MIC. This method is useful for evaluating the efficacies of biocides against a wide range of vegetative organisms, such as bacteria and fungi, and in the development of product preservation. MICs are limited, as many biocides react with organic and inorganic constituents of the growth media and are therefore not available for activity against the test organism; examples of this include oxidizing agents, halogens, and aldehydes. As biocide MICs are generally at relatively low levels, they have limited use in demonstrating the various formulation effects that can enhance the efficacy of an antiseptic or disinfectant. Similar to MIC determination, the minimum microbicidal concentration (e.g., the minimum bactericidal concentration) can be determined by exposing the test organism to biocide or biocidal-product dilutions for a fixed time and then determining at what concentration no growth is observed. These tests are not widely used to evaluate biocidal activity.
Читать дальше














