1 ...7 8 9 11 12 13 ...22
Bile Formation, Secretion and the Enterohepatic Circulation
Bile is a complex secretion that originates from hepatocytes and is modified distally by absorptive and secretory transport systems in the bile duct epithelium. Bile formation by the hepatocytes involves secretion of osmotically active inorganic and organic anions into the canalicular lumen, followed by passive water movement. Bile then enters the gallbladder where it is concentrated or is delivered directly to the bowel.
Bile comprises about 95% water in which are dissolved a number of endogenous constituents, including bile salts, bilirubin, phospholipid, cholesterol, amino acids, steroids, enzymes, porphyrins, vitamins, and heavy metals, as well as exogenous drugs, xenobiotics and environmental toxins [4]. Lipophilic constituents are in solution in mixed micelle composed of BAs, phospholipids, and cholesterol.
Bile is essential for several important functions:
the excretion of potentially harmful exogenous lipophilic substances, as well as the excretion of endogenous substrates such as bilirubin and bile salts;
the digestion and absorption of lipid in the gut by bile salts;
cholesterol homeostasis, by facilitating intestinal cholesterol absorption and, on the other hand, promoting cholesterol elimination;
the excretion of immunoglobulin A (IgA) and inflammatory cytokines, thus protecting the organism from enteric infections;
signaling properties of the BAs in the liver and the intestine, which are mediated by nuclear BA receptors such as FXR, PXR and vitamin D receptor (VDR), as well as by membrane a5b1 integrin, epidermal growth factor receptor, and sphingosine‐1‐phosphate receptor 2.
Our understanding of cholestatic liver diseases has been profoundly advanced by the discovery of nuclear receptors for BA signaling and their role in hepatobiliary excretory function and the adaptive changes counteracting the liver injury caused by retained, potentially toxic, and proinflammatory BAs. Ligand‐activated nuclear receptors such as FXR control a broad range of metabolic processes, including hepatic BA transport and metabolism, lipid and glucose metabolism, drug disposition, as well as liver regeneration, inflammation, fibrosis, cell differentiation, and tumor formation. Moreover, FXR has anti‐inflammatory and immunomodulatory actions and controls intestinal integrity and permeability, as well as gut microbiota. Conversely, the gut microbiota metabolizes BAs, with formation of secondary BAs that, in turn, modulate BA signaling. Based on these broad physiologic effects in the liver and intestine, drugs targeting FXR and TGR5 therefore open important perspectives for pharmacotherapy of cholestatic and metabolic liver disorders, including the complications of liver cirrhosis such as portal hypertension and hepatocellular carcinoma (HCC).
In addition, BAs stimulate glucagon‐like peptide (GLP)‐1 production via TGR5 activation. GLP‐1 is known to promote insulin secretion and thus regulate glucose homeostasis. Because GLP‐1 mimetics and receptor agonists are currently under clinical development and have shown promise in improving glucose homeostasis in diabetes, BA‐based TGR5 agonists may be a potential therapeutic to stimulate GLP‐1 secretion in diabetic patients.
Bile Acid Synthesis and Metabolism
BAs are synthesized from cholesterol. In humans, the “primary” BAs are cholic acid (CA) and chenodeoxycholic acid (CDCA). Before secretion into the bile, both CA and CDCA are conjugated to the amino group of taurine or glycine. Conjugation enhances the hydrophilicity of the BA, the major function of this being to decrease the passive diffusion of BAs across the cell membranes during their transit through the biliary tree and intestine. Therefore, conjugated BAs are absorbed only if a specific membrane carrier is present. The process of bile formation depends on the liver synthesis and the canalicular secretion of BAs. The active transport of BAs across the canalicular membranes of hepatocytes is a primary driving force for bile flow. The majority of the BAs in the intestine are absorbed intact. Approximately 15% are deconjugated by the bacterial flora in the distal small intestine, with the production of “secondary” BAs by the conversion of CA to deoxycholic acid and of CDCA to lithocholic acid. Most of the conjugated and deconjugated BAs are reabsorbed in the distal intestine and undergo enterohepatic circulation that maintains the BA pool. Thus, at least 12 major conjugated primary and secondary bile salt species are contained in human bile, although primary bile salts are usually predominant.
Enterohepatic Bile Acid Circulation
BAs undergo an enterohepatic circulation that depends on active transport systems in the liver and the intestine ( Figure 1.2). More specifically, BAs are excreted from hepatocytes into bile through BSEP/ABCB11 at the bile canaliculus, reabsorbed in the ileum by the apical sodium‐dependent bile salt transporter (ASBT/SLC10A2), and return through the portal blood to the liver, where they are taken up by hepatocytes via the basolateral transport systems NTCP/SLC10A1 for conjugated BAs and OATPs/SLCO/SLC21 family for unconjugated BAs, thus limiting the amount of BA spillover into the systemic circulation. BAs complete the enterohepatic circulation six to eight times a day and are highly efficiently conserved. BAs that escape ileal reabsorption reach the colon, where they are deconjugated and metabolized (e.g. dehydroxylated) by gut microbiota to secondary BAs, which can still be passively absorbed as unconjugated BAs in the colon. Unconjugated BAs are partially reconjugated (and rehydroxylated) during their passage through the liver before being excreted into bile again, which completes their enterohepatic cycle. In addition, BAs are filtered by the glomeruli and then reabsorbed in renal tubules, again limiting their renal loss.
BAs may also cycle between cholangiocytes and hepatocytes through a cholehepatic shunt pathway. Unconjugated BAs induce a greater degree of bile flow per BA molecule excreted in bile. To account for this hypercholeretic effect, it was proposed that unconjugated BAs may be passively absorbed by bile ducts, enter the peribiliary plexus adjacent to intrahepatic bile ducts, and then forwarded to the hepatic sinusoids to be returned to cholangiocytes by hepatocyte secretion. Cholehepatic shunting initiated by passive absorption of non‐ionized bile salt results in the generation of HCO 3 –‐rich hypercholeresis [4,5].
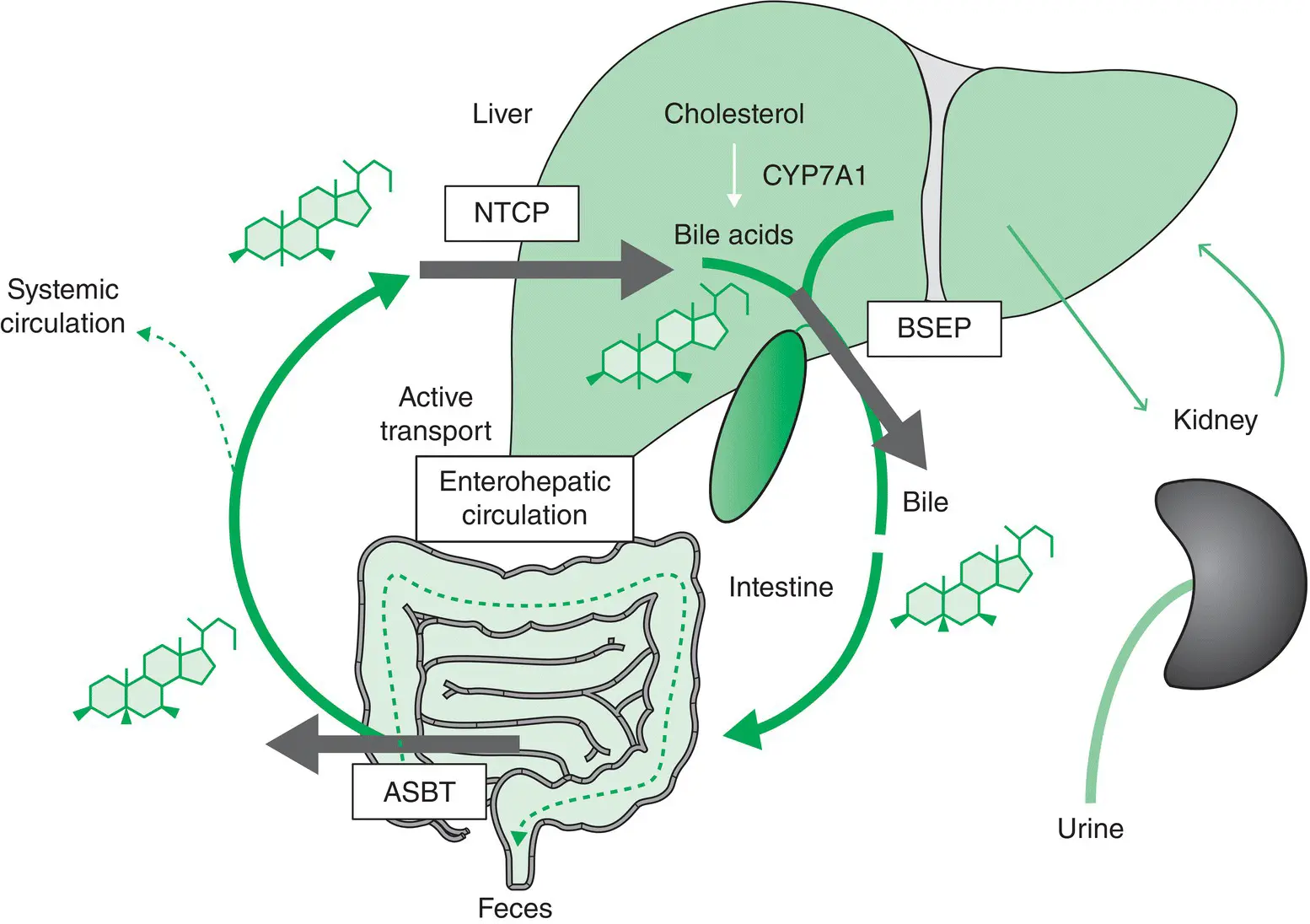
Figure 1.2 Enterohepatic circulation of bile acids (BAs). After hepatic synthesis and biliary secretion, BAs undergo enterohepatic circulation. The BSEP (ABCB11) is the main canalicular transporter for BAs. After reabsorbtion by ASBT/SLC10A1 in the ileum, BAs (and exit through OSTα/β from enterocytes; not shown) are transported back to the liver through the portal blood. Reuptake of conjugated BA from portal blood through NTCP/SLC10A1 (and OATPs for unconjugated BAs; not shown) into the hepatocytes completes the enterohepatic circulation. ASBT, apical sodium‐dependent bile salt transporter; BSEP, bile salt export pump; NTCP, sodium taurocholate cotransporting polypeptide; OATPs, organic anion transporters; OST, organic solute transporter α/β.
Source: Trauner et al . [5]. Reproduced with permission of John Wiley and Sons.
Death and Regeneration of Hepatocytes
Cell Death
Hepatocytes can die because of either necrosis or apoptosis. Necrosis is the loss of plasma membrane integrity with release of the cellular contents locally, which triggers an inflammatory response. Apoptosis is a highly regulated process in which cells that are damaged, senescent or deregulated self‐destruct with a lower release of inflammatory products. Dying cells undergo morphologic modifications including chromatin condensation, nuclear fragmentation, and generation of apoptotic bodies. Furthermore, they express signals on the cell surface that allow macrophage recognition. Apoptosis is essential to avoid the outflow of intracellular contents and to limit the immunologic response against intracellular autoantigens. Nevertheless, apoptotic bodies and fragments can under some circumstances constitute a major source of immunogens in autoimmune diseases that involve the targeting of ubiquitous autoantigens. This has been described in PBC. In the BECs of patients with PBC there is increased DNA fragmentation, implying increased apoptosis, when compared with normal controls. While mitochondrial proteins are present in all nucleated cells, in PBC there is a highly specific multilineage immune response directed to the nominal mitochondrial autoantigenic epitope, the inner lipoyl domain of the E2 subunit of the pyruvate dehydrogenase complex (PDC‐E2) of the BECs. Apoptosis of BECs has been proposed as a potential source of “neoantigens” that could be responsible for activation of autoreactive T lymphocytes or a target for effector cells or antibodies. PDC‐E2 is not only immunologically intact during apoptosis in BECs, but it localizes in the apoptotic bodies of BECs where it is accessible to recognition by anti‐mitochondrial antibodies.
Читать дальше