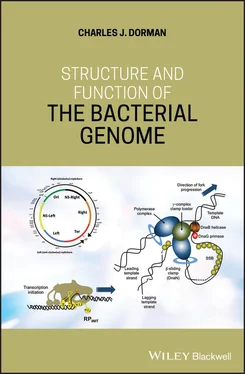
Figure 1.19The multifaceted stringent response. A summary is shown of the processes that are inhibited or enabled by the alarmone (p)ppGpp. (a) (p)ppGpp and DksA affect a stringently regulated promoter that contains a G+C‐rich discriminator sequence negatively. Genes encoding rRNA or tRNA are in this category. (b) In contrast, (p)ppGpp and DksA affect a promoter with an A+T‐rich discriminator positively. Genes involved in amino acids biosynthesis are in this category. (c) The (p)ppGpp alarmone biases the selection of sigma factors by RNA polymerase away the RpoD housekeeping sigma factor and towards sigma factors that are required for various stress responses. (d) The initiation of chromosome replication is inhibited by (p)ppGpp. (e) Translation initiation and translation elongation are affected negatively because (p)ppGpp has an inhibitory influence on Initiation Factor 2 (IF2) and on the translation elongation factor, EF, respectively.
1.40 FIS and DNA Topology
The involvement of FIS in SIDD‐based regulatory mechanisms has already been described. The promoter of the dusB‐fis operon is subject to transcriptional stimulation by DNA negative supercoiling (Schneider et al. 2000) in addition to being auto‐repressed by FIS and controlled negatively by the stringent response (Ninnemann et al. 1992). At a global level, the FIS protein is intimately associated with the general management of DNA topology in the bacterial cell. It represses the transcription of the gyrA and gyrB genes in E. coli (Schneider et al. 1999) and Salmonella (Keane and Dorman 2003) and has a complicated relationship with the promoters of the topA gene, where its influence is conditional on factors such as oxidative stress (Weinstein‐Fischer and Altuvia 2007). Although E. coli and Salmonella have distinct DNA supercoiling set points, with Salmonella DNA being more relaxed than in E. coli (Champion and Higgins 2007), this distinction is dependent on the presence of FIS (Cameron et al. 2011). Thus, the pattern of expression of the topoisomerases responsible for negative supercoiling (DNA gyrase) and relaxation (Topo I) of DNA is modulated by FIS. The activities of these topoisomerases is also affected by FIS because the protein influences their access to DNA by binding to it: since FIS prefers to bind to DNA with intermediate levels of negative supercoiling it acts to preserve this topological form (Schneider et al. 1997; Cameron and Dorman 2012).
In order to exert its influence on DNA topology, FIS must be present in the cell. This restricts its influence to the early stages of exponential growth when it is most abundant (Schneider et al. 1997). An exception has been discovered in bacteria growing under micro‐aerobic conditions: here FIS levels are sustained into the stationary phase of growth (Cameron et al. 2013; O Cróinín and Dorman 2007). This may be of special significance in environments such as the mammalian gut epithelial surface where FIS‐dependent gene expression is required for colonisation and invasion (Falconi et al. 2001; Kelly et al. 2004; Prosseda et al. 2004; Rossiter et al. 2015).
1.41 Ferritin‐Like Dps and the Curved‐DNA‐binding Protein CbpA
While FIS is associated with the early stages of rapid exponential growth, the Dps (DNA‐binding protein from starved cells) and CbpA proteins exhibit the polar opposite expression pattern and are seen predominantly in stationary phase (Ali Azam and Ishihama 1999). Both are NAPs and Dps has been studied in the most detail. CbpA expression is prevented during exponential growth by the FIS protein. FIS, which is abundant in this period of the growth cycle, binds and represses the activity of an RpoD‐dependent promoter that is located in a gene ( yccE ) adjacent to cbpA that is partly responsible for cbpA transcription in stationary phase. A second promoter immediately upstream of cbpA depends on RpoS, a sigma factor that is only available in stationary phase or in stressed cells ( Figure 1.20) (Chintakayala et al. 2013). CbpA forms dimers in solution and aggregates following binding to DNA, forming nucleoprotein complexes similar to those produced by the Dps NAP (Cosgriff et al. 2010). The preferred DNA targets of CbpA are A+T‐rich and intrinsically curved; this protein has a marked preference for binding within the Ter macrodomain of the chromosome, a zone of high DNA curvature and with a high A+T content (Chintakayala et al. 2013). CbpA binds at the minor groove of DNA and cbpA mutants display aberrant DNA topology, observations that are consistent with a role in organising the DNA in the Ter macrodomain during stationary phase (Chintakayala et al. 2013, 2015).
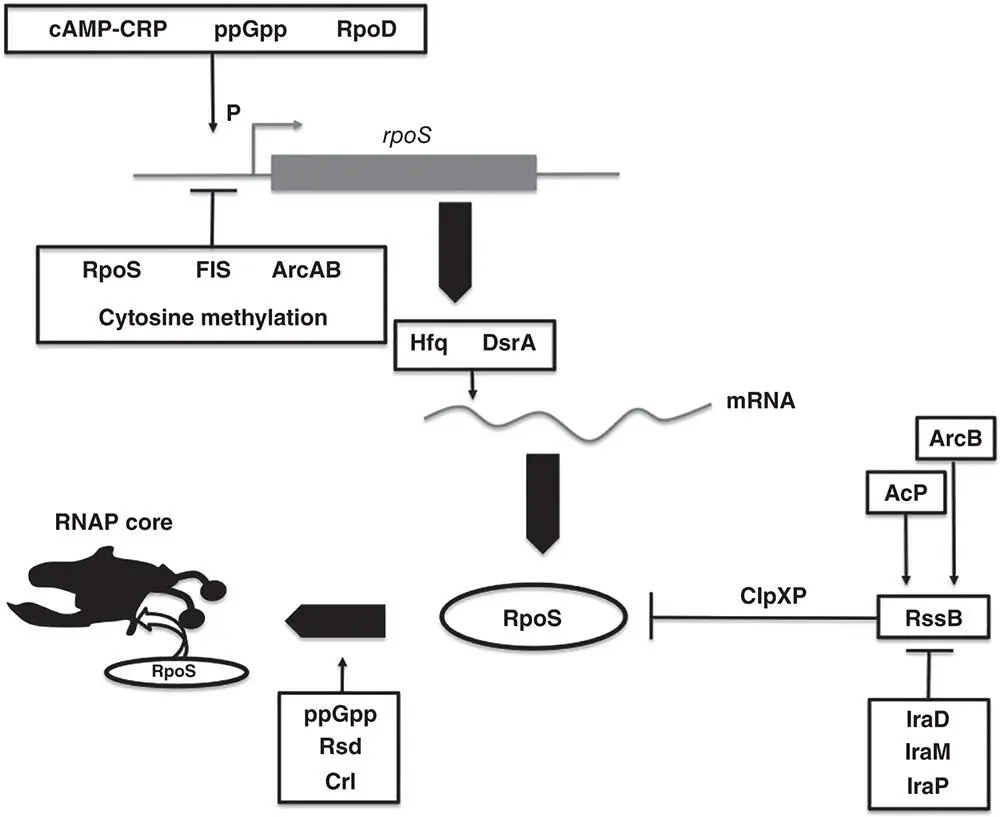
Figure 1.20The stress and stationary phase sigma factor, RpoS. The rpoS gene is influenced at the transcriptional level by several factors. The glucose‐sensitive cAMP‐CRP complex and the stringent‐response signal, ppGpp, act at the RpoD‐dependent promoter to enhance rpoS transcription; production of RpoS is enhanced in mutants deficient in cytosine methylation (see Kahramanoglou et al. 2012). It is important to note that rpoS is expressed under all growth conditions and that the principal regulatory effects are imposed at the level of RpoS protein stability; rapidly growing bacteria have few copies of RpoS and non‐growing bacteria have many. The expansion of the population of RpoS proteins occurs in bacteria when growth is slowed or stopped due to stress. The stress can be physical or chemical in nature. Once transcribed, RpoS mRNA is translated poorly due to the formation of secondary structures that sequester the translation initiation signals. These stem‐loops are eliminated by the DksA sRNA that binds to the 5′ end of the mRNA in the presence of the Hfq RNA chaperone protein. DksA also controls the translation of the hns transcript, albeit negatively due to sequestration of the translation initiation signals. The RpoS protein is degraded by the ClpXP protease. Proteolytic cleavage of RpoS is enhanced by the adaptor protein, RssB. RssB activity is in turn modulated negatively by the sRNAs IraD, IraM, and IraP in response to stresses that impede the growth of the bacterium. In Salmonella, IraM is called RssC. RssB activity is controlled in response to changes to oxygen supply (ArcB) and carbon levels (Acp). The sigma factor must compete with RpoD and other sigma factors for access to the core RNA polymerase and it is assisted in doing so by ppGpp, Crl, and the anti‐sigma factor, Rsd.
Dps is dodecameric in E. coli and has ferritin‐like properties (Grant et al. 1998). This protein accumulates in stationary phase bacteria and was found initially to protect the genomic DNA from oxidative damage (Almirón et al. 1992; Martinez and Kolter 1997). It does not impede transcription, despite being an abundant DNA‐binding protein (Janissen et al. 2018). Dps was subsequently discovered also to afford protection against gamma radiation, ultraviolet light, copper and iron toxicity, heat stress, and pH shock (Algu et al. 2007; Jeong et al. 2008; Nair and Finkel 2004). It also protects DNA from cleavage by restriction enzymes (Janissen et al. 2018).
Although Dps is usually grouped with the NAPs (Ali Azam and Ishihama 1999), its relationship with DNA has been difficult to determine with precision. This protein can form a co‐crystal with DNA, perhaps accounting for its ability to protect the chromosome from damage in stressed cells (Wolf et al. 1999). Through the application of SELEX (systematic evolution of ligands by exponential enrichment) to E. coli , a DNA sequence has been identified that seems to contain the elements of a Dps‐binding site (Ishihama et al. 2016). A closely related motif has been detected in E. coli by chromatin immunoprecipitation on chip (Antipov et al. 2017). These Dps‐binding sites overlap with those of other NAPs, leading to speculation that Dps supplies the genome architectural functions of those proteins (such as FIS) that are no longer expressed as the bacterium enters stationary phase (Antipov et al. 2017). Alternatively, Dps binding and the binding of other NAPs, such as IHF, may alternate depending on the environmental conditions that accompany entry of the bacterium into stationary phase (Lee et al. 2015).
Читать дальше