1.35 DNA Bending Protein Integration Host Factor (IHF)
IHF is essential both for the integration and the excision of bacteriophage lambda into/from the E. coli chromosome (Bushman et al. 1984; Seah et al. 2014) ( Figure 1.17). These site‐specific recombination reactions are catalysed by the tyrosine integrase Int (Craig and Nash 1983; Han et al. 1994; Hoess et al. 1980; Kikuchi and Nash 1979; Tong et al. 2014). IHF is usually expressed as a heterodimer composed of an alpha and a beta subunit encoded by the ihfA and ihfB genes, respectively, which are located at different places on the chromosome (Haluzi et al. 1991; Mendelson et al. 1991; Miller and Friedman 1980; Nash et al. 1987). IHF has strict DNA sequence requirements for binding and its binding sites typically are located in A+T‐rich DNA (Miller and Friedman 1980). The protein inserts a looped beta strand with a proline amino acid at its apex into the minor groove of the DNA at the target site, producing a distortion that causes the DNA duplex to bend (Engelhorn and Geiselmann 1998; Engelhorn et al. 1995; Rice et al. 1996; Sun et al. 1996; Vivas et al. 2012) ( Figure 1.18). The combined effects of the bends introduced by each subunit is to create a turn of up to 180° in the pathway taken by the DNA helix (Rice et al. 1996; Sugimura and Crothers 2006). This allows IHF to play important roles in nucleoid architecture, chromosome replication, site‐specific recombination, transposition, plasmid maintenance, and transcription regulation (Biek and Cohen 1989; Crellin et al. 2004; Dorman and Bogue 2016; Prieto et al. 2012; Ryan et al. 2004; Saha et al. 2013; Swinger and Rice 2004). In terms of amino acid sequence, protein structure, and subunit composition, IHF is a close relative of the HU NAP. Both proteins have an αβ heterodimeric structure and all four proteins have similar amino acid sequences (Dey et al. 2017). IHF appears to be a specialist member of the HU superfamily of DNA‐binding proteins, a group that includes relatives encoded by the human genome that contribute to chromosome partitioning (Burroughs et al. 2017). Although IHF is usually considered as acting in an αβ heterodimeric form, transcriptomic studies carried out in Salmonella indicate that bacteria expressing just the α, just the β, and both α and β subunits, are altered in expression of distinct‐yet‐overlapping groups of genes, implying that IHF can form homodimers that are biologically active (Mangan et al. 2006). Indeed, previous investigations have found that IHF homodimers have DNA‐binding activity (Hiszczynska‐Sawicka and Kur 1997; Werner et al. 1994; Zablewska and Kur 1995; Zulianello et al. 1994). When reading the older IHF literature, the reader should be aware that in 1996 the names of the genes were changed from himA and himD (where ‘him’ referred to H.I. Miller) to ihfA and ihfB , respectively (Weisberg et al. 1996).
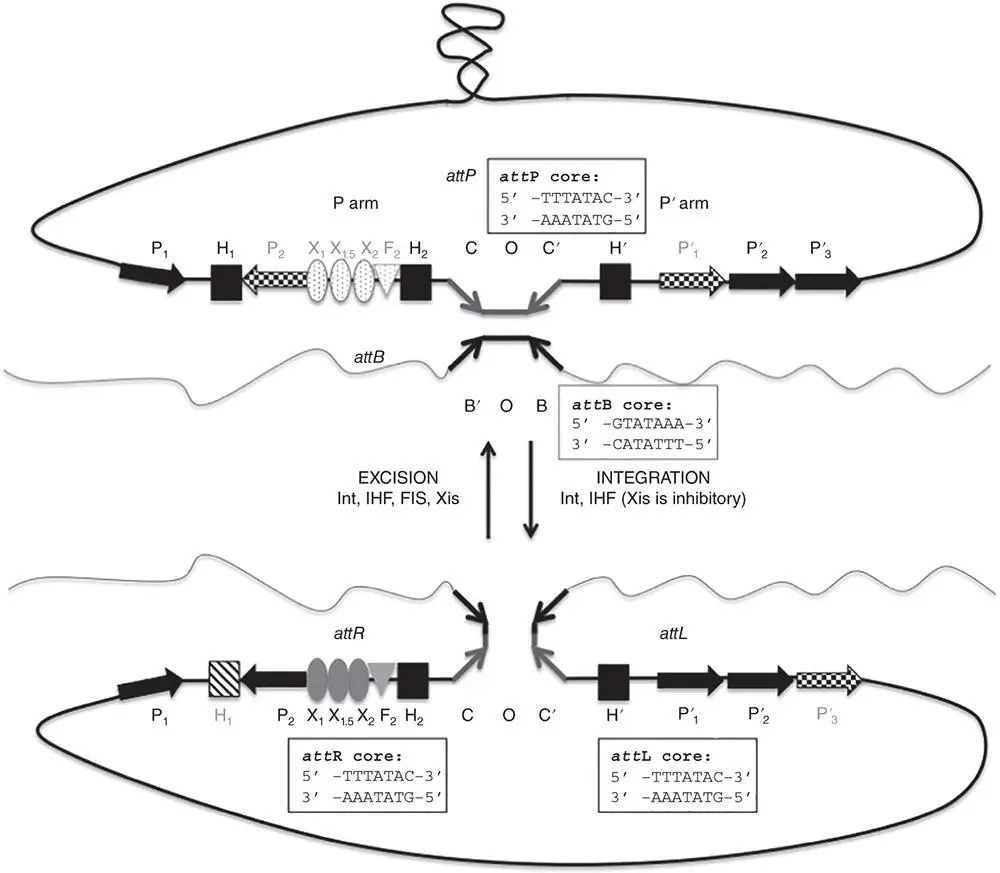
Figure 1.17Integration of bacteriophage lambda at the lambda attachment site on the E. coli chromosome and excision of the prophage. Integration and excision are catalysed by the phage‐encoded Int tyrosine integrase. In the integration step, the recombining sites at attP (on the phage) and attB (on the bacterial chromosome) undergo intermolecular site‐specific recombination to generate the lambda prophage. Excision recreates attB and attP from attL and attR, the direct repeats that form the boundaries between the prophage and chromosomal DNA. The nucleotide sequences of each of these four elements are shown in the figure. In addition to the Int recombinase, the integration step requires the architectural protein, integration host factor, IHF. Excision also requires IHF together with the phage‐encoded directionality factor, Xis, and the host‐encoded FIS protein. Xis is inhibitory to integration and FIS has a stimulatory effect on excision (see Ball and Johnson 1991; Seah et al. 2014). Binding sites for these proteins are represented by labelled arrows (P, Int), squares (H, IHF), ovals (X, Xis), and a triangle for a FIS (F) binding site. Binding sites in the P′ arm of the free circular phage genome have primes added to their designations and these are retained in the attL segment of the prophage. Not every protein‐binding site is occupied in each reaction. Occupied sites have a solid filling while unoccupied sites have a speckled or hatched filling. During integration, Int‐binding sites P 1, P′ 2, and P′ 3are occupied while P 2and P′ 1are not; all three IHF‐binding sites are occupied but the XIS and FIS sites are vacant. During excision, Int sites P 1, P 2, P′ 1, and P′ 2are occupied, as are IHF sites H 2and H′ and all of the Xis and FIS sites; IHF site H 1and Int site P 3are vacant.
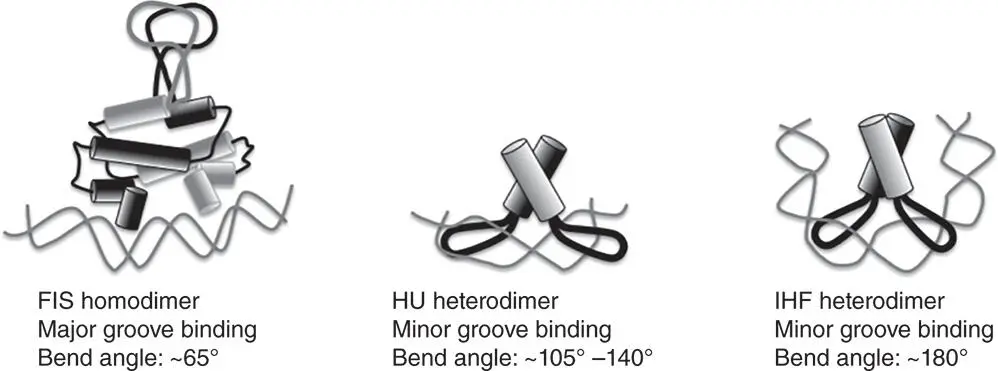
Figure 1.18The interactions of the nucleoid‐associated proteins FIS, HU, and IHF with DNA. The FIS protein is homodimeric, while HU and IHF are heterodimeric. HU and IHF are closely related at the level of amino acid sequence and the alpha and beta subunits of each protein are similar in sequence and secondary structure. The tertiary structures of HU and IHF are also quite similar, as their modes of binding to DNA. Each inserts a beta sheet from each subunit into the minor groove of its DNA target. IHF differs from HU in having a strict nucleotide sequence requirement for binding and in making more contacts with the DNA at its binding site. IHF also induces a much greater bend angle in the DNA. The bends on either side of the HU‐DNA complex are non‐coplanar. FIS binds in the major groove of DNA using an alpha helix, one of four alpha helices found in each FIS monomer. The protein uses an induced fit binding mechanism that compresses the minor groove lying between the two sites of insertion of the alpha helices in the major groove. FIS binds to a variety of sites with differing binding affinities; a Logo has been assembled that summarises the chief sequence characteristics of the highest affinity sites (see Stella et al. 2010).
1.36 HU, a NAP with General DNA‐binding Activity
The HU protein from E. coli is the founder member of a superfamily of related NAPs found throughout the prokaryotic world and beyond (Burroughs et al. 2017). HU interacts with DNA in the minor groove and this encourages the bound DNA to follow a looped path ( Figure 1.18). This property helps HU to overcome the resistance of the DNA to loop formation by overcoming DNA's intrinsic stiffness (Johnson et al. 1986). HU‐assisted loop formation contributes to the formation of nucleoprotein complexes involved in the control of transcription and site‐specific recombination (Haykinson and Johnson 1993; Semsey et al. 2004). It also has RNA‐binding activity, enabling it to influence translation (Balandina et al. 2001).
Each HU subunit inserts a beta sheet with an apical proline amino acid into the minor groove of the DNA at the binding site, inducing the DNA to bend ( Figure 1.18). The bend angle is typically in the range of 105°–140° and bends are not coplanar, having a dihedral angle that is consistent with the path taken in negatively supercoiled DNA (Swinger et al. 2003). The flexibility in the bend angle, coupled with the absence of a strict nucleotide sequence for DNA binding, may allow HU to participate as an architectural component in a wide variety of DNA‐based transactions.
Читать дальше