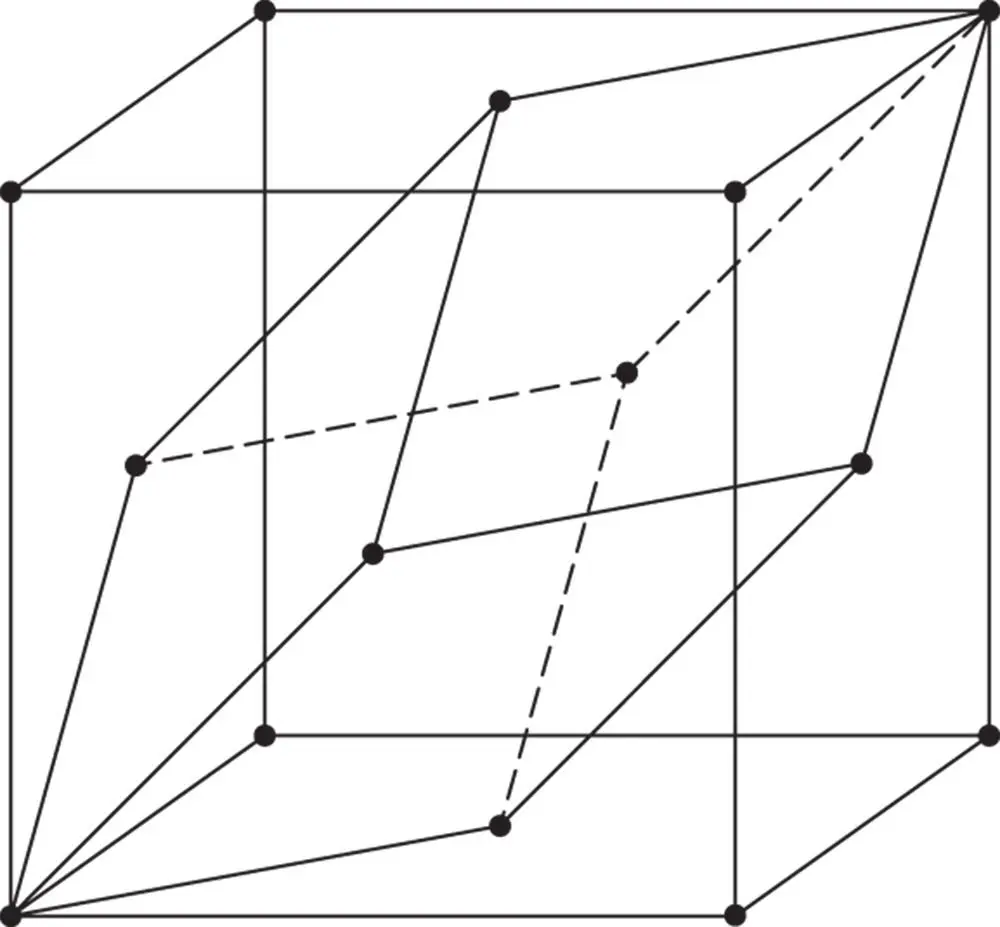
Figure 1.31The relationship between the primitive unit cell and the conventional cell in the face‐centred cubic lattice
The large non‐primitive unit cell in Figures 1.19m and 1.31is the face‐centred cubic lattice, which can be designated F . It contains four lattice points. These are at the corners and centres of each of the faces.
When h in Figure 1.28becomes equal to 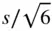 , the primitive unit cell of the R lattice becomes a cube with α = 90°. This is the cubic primitive lattice P shown in Figure 1.19l, containing lattice points at the corners of the cubic unit cell.
, the primitive unit cell of the R lattice becomes a cube with α = 90°. This is the cubic primitive lattice P shown in Figure 1.19l, containing lattice points at the corners of the cubic unit cell.
Lastly, if in Figure 1.28 h takes the value 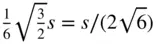 , the angle α is equal to 109.47° (= 180° − 70.53°) = cos −1(−1/3). The lattice formed by such an array of points also contains four threefold axes of symmetry. The conventional unit cell of this lattice is shown in Figure 1.19n. It is a cube with lattice points at the cube corners and one at the centre. It can be designated I , the cubic body‐centred lattice. The relationship between the doubly primitive unit cell shown in Figure 1.19n and the primitive unit cell, which is a rhombohedron with axial angles of 109.47°, is shown in Figure 1.32.
, the angle α is equal to 109.47° (= 180° − 70.53°) = cos −1(−1/3). The lattice formed by such an array of points also contains four threefold axes of symmetry. The conventional unit cell of this lattice is shown in Figure 1.19n. It is a cube with lattice points at the cube corners and one at the centre. It can be designated I , the cubic body‐centred lattice. The relationship between the doubly primitive unit cell shown in Figure 1.19n and the primitive unit cell, which is a rhombohedron with axial angles of 109.47°, is shown in Figure 1.32.
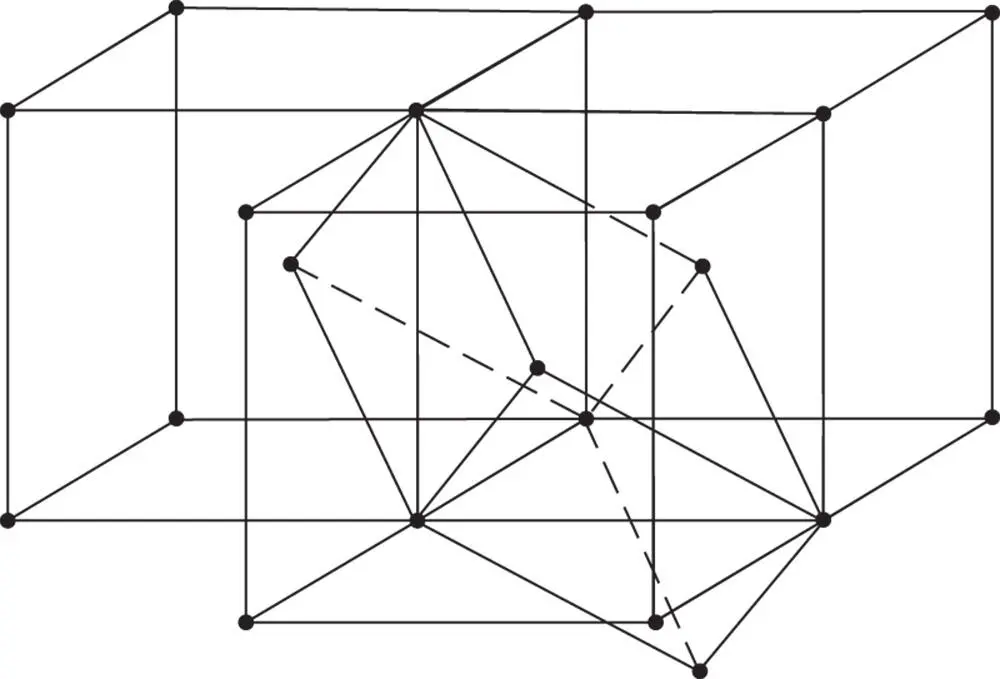
Figure 1.32The relationship between the primitive unit cell and the conventional cell in the body‐centred cubic lattice
We have just described the three lattices consistent with the possession of four threefold axes of rotational symmetry. They are shown together in Figures 1.19l, m and n. The unit cell of each can be taken as a cube with a = b = c ; α = β = γ = 90°. The primitive cell contains one lattice point, the face‐centred cell four, and the body‐centred cell two.
The unit cells of the 14 Bravais space lattices are shown together in Figure 1.19. All crystals possess one or other of these lattices, with an identical atomic motif associated with each lattice point. In some crystals a single spherically symmetric atom is associated with each lattice point. In this case the lattice itself possesses direct physical significance because the lattice and the crystal structure are identical. In other cases the lattice is a very convenient framework for describing the translational symmetry of the crystal. If the lattice is given and the arrangement of the atomic motif about a single lattice point is given, the crystal structure is fully described. The lattice is the most important symmetry aspect for describing the properties of imperfections in crystals.
The material in Appendix 1 may assist in some of these exercises .
1 1.1Select any convenient point on the plane pattern appearing on the diagram below and mark all the corresponding points, thus indicating the lattice.Outline the unit cell in several different ways, mark in the x‐ and y‐axes in each case, and measure the cell dimensions a, b and γ in each case.Draw a line parallel to MN through any one lattice point and add all the lines of this set. Determine the indices of this set of lines for each of your different choices of unit cell.Repeat (c) for the set of lines parallel to PQ.Figure for Problem 1.1
2 1.2 Rutile, TiO2, has a = b = 4.58 Å, c = 2.95 Å and α = β = γ = 90°. The atomic coordinates are:O: u, u, 0; −u, −u, 0; + u, – u, ; − u, + u, , where u = 0.31Draw an accurate projection of a unit cell on the plane containing the x‐ and y‐axes.Determine the number of formula units per cell.Find the number of oxygen atoms surrounding each titanium atom. Calculate the interatomic distances Ti–O for the titanium atom at .
3 1.3 The unit cells of several orthorhombic crystals are described below. What is the Bravais lattice of each?Two atoms of the same kind per unit cell located at: Four atoms of the same kind per unit cell located at:Two atoms of one kind per unit cell located at , , ; , , and two of another kind located at , , ; , , .
4 1.4 Show with a sketch that a face‐centred tetragonal lattice is equivalent to a body‐centred tetragonal lattice in a different orientation. Why are there no tetragonal C, monoclinic I, cubic C, and hexagonal I Bravais lattices?A crystal structure is proposed for which a = c ≠ b; α = γ = 90°; β > 90°. Close examination of the proposed structure shows that it has a monoclinic I lattice. Specify a more conventional description of the lattice type and describe the relationship of the sides of the unit cell for this new lattice type in terms of the monoclinic I unit cell.
5 1.5 The spinel MgAl2O4 has a = b = c = 8.11 Å and α = β = γ = 90°. Crystals show the faces (111), (), () and () and the faces parallel to these, namely (), (), () and (). Such crystals look like regular or distorted octahedra.Find the zone axis symbol (indices) of the zone containing (111) and (). Which of the other faces listed above also lie in this zone?Show that the zone containing (111) and () also contains (001) and (110).Sketch the projection of the lattice on (001). Draw in the trace of the (110) lattice plane and the normal to the plane through the origin. Find the perpendicular distance of the first plane of this set from the origin in terms of the cell side.Draw the section of the lattice through the origin which contains the normal to (110) and the normal to (001). Mark on this section the traces of the (111), (), () and () planes. Measure the angles between adjacent planes.
6 1.6 Find the angle between [111] and the normal to (111) in (a) a cubic crystal and (b) a tetragonal one with a = 5.67 Å, c = 12.70 Å.
7 1.7 Show that the faces (111), (231), and () all lie in a zone. (a) Find the zone symbol (indices) of this zone and (b) calculate the angle between this zone and the [100] zone in the cubic system.
8 1.8 In a tetragonal crystal CuFeS2, the angle between (111) and () is 108.67°. Calculate (a) the ratio of the axial lengths and (b) the interzone angle [236]^[001].
9 1.9 Following the method of Section 1.6, determine the angles between the rotational axes in the axial combinations of (a) two triads and a diad and (b) a tetrad, a triad and a diad. Draw a sketch to show the arrangement of the symmetry operations in each case.
10 1.10 Show that (a) successive combinations of rotations about two different tetrad axes are unable to be expressed as a rotation about a single triad axis in crystals, and (b) successive combinations of rotations about two different triad axes are unable to be expressed as a rotation about a single tetrad axis in crystals.
11 1.11 The unit cell of a two‐dimensional lattice has a = b, γ = 120°. Dispose three atoms about each lattice point in different ways so that the two‐dimensional crystal can show the following symmetries in turn: (a) a sixfold axis with mirror planes parallel to it, (b) a threefold axis, (c) a diad axis at the intersection of two mirror planes, (d) a twofold axis, and (e) a onefold axis.
12 1.12 CdI2 and CdCl2 both belong to the trigonal crystal system. The former has a primitive hexagonal lattice and the latter a rhombohedral lattice. The coordinates of the atoms associated with each lattice point are given below, using a hexagonal unit cell for both crystals:Draw plans of the two structures on (001) and outline on your diagram for CdCl2 the projections of the cell edges of the true rhombohedral primitive unit cell.
Читать дальше


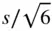 , the primitive unit cell of the R lattice becomes a cube with α = 90°. This is the cubic primitive lattice P shown in Figure 1.19l, containing lattice points at the corners of the cubic unit cell.
, the primitive unit cell of the R lattice becomes a cube with α = 90°. This is the cubic primitive lattice P shown in Figure 1.19l, containing lattice points at the corners of the cubic unit cell.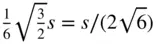 , the angle α is equal to 109.47° (= 180° − 70.53°) = cos −1(−1/3). The lattice formed by such an array of points also contains four threefold axes of symmetry. The conventional unit cell of this lattice is shown in Figure 1.19n. It is a cube with lattice points at the cube corners and one at the centre. It can be designated I , the cubic body‐centred lattice. The relationship between the doubly primitive unit cell shown in Figure 1.19n and the primitive unit cell, which is a rhombohedron with axial angles of 109.47°, is shown in Figure 1.32.
, the angle α is equal to 109.47° (= 180° − 70.53°) = cos −1(−1/3). The lattice formed by such an array of points also contains four threefold axes of symmetry. The conventional unit cell of this lattice is shown in Figure 1.19n. It is a cube with lattice points at the cube corners and one at the centre. It can be designated I , the cubic body‐centred lattice. The relationship between the doubly primitive unit cell shown in Figure 1.19n and the primitive unit cell, which is a rhombohedron with axial angles of 109.47°, is shown in Figure 1.32.











