TYPE IV SECRETION SYSTEMS
Type IV secretion systems(T4SS) utilize a secretin-like protein (VirB9) that forms a β-barrel channel in the outer membrane and extends into the periplasm, where it makes contact with proteins in the inner membrane. However, unlike true secretins, it seems to require another outer membrane protein, VirB7, to make a channel. The VirB9 protein is covalently attached to the VirB7 protein, which in turn is covalently attached to the lipid membrane, making the structure very stable. A coupling protein (VirD4) binds specific proteins and targets them to the channel. The energy for secretion comes from the cleavage of ATP or GTP in the cytoplasm by channel-associated proteins ( Figure 2.39).
T4SS are discussed in chapters 5and 6, because they are also involved in DNA transfer during conjugation and transformation. The T-DNA transfer system of Agrobacterium tumefaciens has served as the prototype T4SS and is the one about which the most is known and to which all others are compared. Accordingly, the genes and proteins of other T4SS are numbered after their counterparts in the T-DNA transfer system, named the vir genes because of their role in virulence in plants. Some of the genes in the T-DNA transferred into plant cells cause growth of the plant cell, leading to the formation of tumors called crown galls. Others trigger the plant cells to make unusual compounds, called opines, that can be used by the bacterium as a carbon, nitrogen, and energy source (see Box 5.1). Like other T4SS, the Agrobacterium system also directly injects proteins into the plant cell, which makes it a bona fide protein secretion system.
TYPE V SECRETION SYSTEMS: AUTOTRANSPORTERS
All of the secretion systems discussed above use some sort of structure formed of β-sheets assembled into a ring called a β-barrel to get them through the outer membrane. Some of these β-barrels are part of the secretion apparatus itself, while others, like TolC, are recruited from other functions in the cell. However, in type V systems, secreted proteins carry their own β-barrel with them in the form of a domain of the protein that can create a β-barrel when it gets to the outer membrane. These proteins are called autotransportersbecause they transport themselves.
The mechanism used by autotransporters is illustrated in Figure 2.40, which also shows their basic structure. Most autotransporters consist of four domains, the translocator domain at the C terminus that forms a β-barrel in the outer membrane, an adjacent flexible linker domain (not shown) that may extend into the periplasm, a passenger domain that contains the functional part of the autotransported protein, and sometimes a protease domain that may cleave the passenger domain off the translocator domain after it passes through the channel formed by the translocator domain.
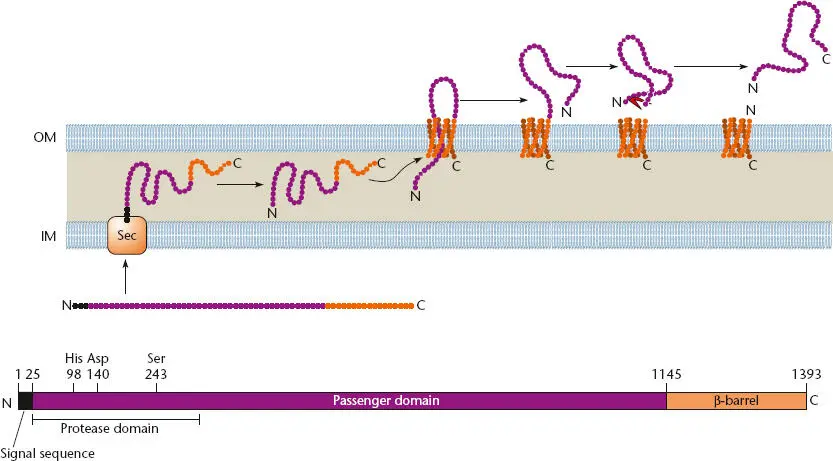
Figure 2.40 Structure and function of a typical autotransporter. A Haemophilus influenzae adhesin is shown; the length in amino acids of each domain, where known, is indicated by the number above the structure, as are some of the important amino acids in the protease domain. The transporter domain at the C terminus that forms a β-barrel in the outer membrane is shown in orange; the passenger domain and the protease domain that cleaves the passenger domain off the transporter domain outside the cell are shown in purple. The flexible linker domain is not indicated. The signal sequence that is cleaved off when the protein passes through the SecYEG (Sec) channel in the inner membrane is shown in black. OM, outer membrane; IM, inner membrane. Modified from Surana NK, Cotter SE, Yeo H-J, et al, in Waksman G, Caparon M, Hultgren S (ed), Structural Biology of Bacterial Pathogenesis (ASM Press, Washington, DC, 2005).
Autotransporters are typically transported through the inner membrane using the SecYEG channel, so they have a signal sequence that is cleaved off as they pass into the periplasm. Their translocator domain then enters the outer membrane, where it forms a 12-stranded β-barrel. This assembly does not occur by itself but requires the same accessory factors used for the assembly of many secretins, including the periplasmic chaperone, Skp, and the Bam complex (see above). The flexible linker domain guides the passenger domain into and through the channel to the outside of the cell. The passenger domain can then be cleaved off by its own protease domain or remain attached to the translocator domain and protrude outside the cell, depending on the function of the passenger domain. The source of the energy for autotransportation is unclear, since, as mentioned, there is no ATP or GTP in the periplasm and the outer membrane does not have a membrane potential. One possibility is that the autotransporter arrives at the periplasm in a “cocked” or high-energy state that drives its own transport.
The prototypical autotransporter is the immunoglobulin A protease of Neisseria gonorrhoeae . It is involved in evading the host immune system by cleaving IgA antibodies on mucosal surfaces. Most known autotransporters are large virulence proteins that perform various roles in bacterial pathogenesis or in helping to evade the host immune system. The IcsA protein of Shigella flexneri , a cause of bacterial dysentery, is localized to the outer membrane, where it recruits a host actin-regulating protein, which in turn recruits another host complex that polymerizes host actin into filaments, pushing the bacterium through the eukaryotic cell cytoplasm as part of the infection mechanism, a process called actin-based motility.
Chaperone-Usher Secretion
Chaperone-usher secretionis related to type V secretion. This type of secretion is often used to assemble some types of pilins on the cell surface, such as the P pilus of uropathogenic E. coli . The secretion system consists of three proteins, a β-barrel-forming protein in the outer membrane called the usher, a periplasmic protein called the chaperone, and the pilin subunit to be assembled on the cell surface. The pilin protein is transported through the inner membrane by the SecYEG channel and therefore has a cleavable signal sequence. Once in the periplasm, the pilin protein is bound by the dedicated periplasmic chaperone. However, rather than merely helping it fold like other chaperones, this chaperone actually contributes a strand to the pilus protein that completes one of the folds of the pilin protein and makes it much more stable (see Waksman and Hultgren, Suggested Reading). The complex of pilin protein and chaperone is then targeted to the usher channel in the outer membrane, where it is assembled into the growing pilus. Again, there is the problem of where the energy for pilus assembly comes from, since the assembly of the pilus occurs at the inner face of the outer membrane after the pilin protein has been transported through the inner membrane and cytoplasm. One idea is that the periplasmic chaperone holds the pilin protein in a high-energy state, and its eventual folding at the usher drives the assembly process.
TYPE VI SECRETION SYSTEMS
Type VI secretion systems (T6SS) are very large, with up to 21 proteins encoded within the gene cluster, 12 of which are highly conserved and are thought to play structural roles in the secretion apparatus. At least some of the others unique to each system may encode effectors specifically transported by that system. An intriguing observation has been made that some of the highly conserved proteins structurally resemble components of phage tails (see chapter 7). The highly conserved protein Hcp resembles gp19, the major tail protein of phage T4, and another, VgrG, resembles the syringe of the baseplate of phage T4, consisting of gp27 and gp5. This has led to speculation that T6SS are derived from phage genes and act as inverted phage tails that now eject proteins from the bacterial cell instead of injecting DNA (and sometimes proteins) into the cell.
Читать дальше












