7.17.3 Haploids from wide crosses
Certain specific crosses between cultivated and wild species are known to produce haploids. Well‐established systems include the interspecific crosses between Hordeum vulgare (2 n = 2x = 14, VV ) × Hordeum bulbosum (2 n = 2x = 14, BB ), commonly called the bulbosum method, and also wheat × maize crosses. The bulbosum method is illustrated in Figure 7.1. The F 1zygote has 2 n = 2x = 14 ( 7 V + 7B ). However, during the tissue culture of the embryo, the bulbosum chromosomes are eliminated, leaving a haploid (2 n = x = 7 V ). This is then doubled by colchicine treatment to obtain 2 n = 2x = 14 VV .
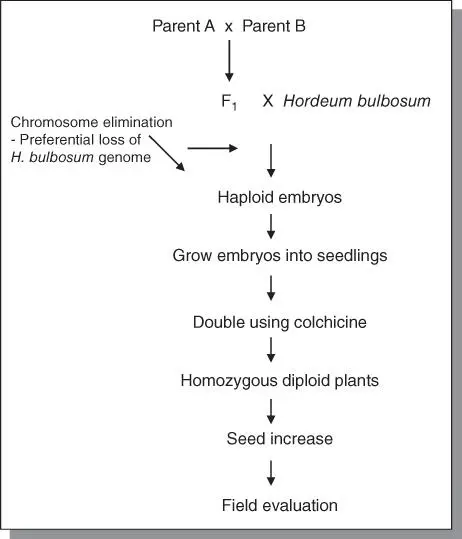
Figure 7.1Generating haploids in barley by the bulbosom method.
Researchers exploit haploidy generally by doubling the chromosome number to create a cell with the double dose of each allele (homozygous).
Inbred lines are homozygous genotypes produced by repeated selfing with selection over several generations. The technique of doubled haploidsmay be used to produce complete homozygous diploid lines in just one year (versus more than four years in conventional breeding) by doubling the chromosome complement of haploid cells. Such doubling may be accomplished in vivo naturally or through crossing of appropriate parents, or in vitro , through the use of colchicine. The success of doubled haploids as a breeding technique depends on the availability of a reliable and efficient system for generating haploids and doubling them in the species.
Doubled haploids have been successfully used in breeding species in which efficient haploid generation and doubling systems have been developed. These include canola, barley, corn, and wheat. Additionally, doubled haploids are used to generate general genetic information that can be applied to facilitate the breeding process. Such information includes gene action and interaction, estimating the number of genetic variances, calculating combining abilities, and detection of gene linkages, pleiotropy, and chromosome locations. Haploids are used in mutation studies (recessive mutants are observable instantly) and in selecting against undesirable recessive alleles.
The first step in using doubled haploids in breeding is identifying the source of haploids.
Natural SourcesHaploids originate in nature through the phenomenon of parthenogenesis (gamete formation without fertilization). The haploids may be maternal or paternal in origin. It is estimated that haploids occur in corn at the rate of 1 in 1000 diploids, 99% of which arise from parthenogenesis of maternal origin. Spontaneous doubling occurs in corn at the rate of 10% of haploids developed. The key is distinguishing between haploid and diploid plants. A marker system for this purpose was first developed by S.S. Chase based on seedling color, purple plants being encoded by the dominant gene (P) while normal green plants are recessive (p). A cross of F1(pp) × PP would yield 999Pp (purple diploids) and 1 pp (green haploid). Another marker used is the purple aleurone color.To use this marker system, the breeder should cross a heterozygous female to a male with marker genes. The seed from those with dominant endosperm marker of the male is saved and planted, discarding seedlings with the dominant male marker. Next, cytological evaluation of plants with the recessive female marker (by root tip squash) is conducted. The haploid plants are retained and grown in the greenhouse or field, and self‐pollinated to produce diploids.
Artificial sourcesHaploid production through interspecific and intergeneric crosses is in use, one of the most well‐known being the barley system (previously discussed). After doubling the chromosome, the diploid plants are grown to maturity. Seeds are harvested for planting plant rows. Because diploids produced by this method are normally completely homozygous, there is no need for growing segregating generations as obtains in conventional programs.
Advantages and disadvantages
The technique of doubled haploids has certain advantages and disadvantages, including the following:
AdvantagesComplete homozygosity is attainable in a shorter period.Duration of the breeding program can be reduced by several (two to three) generations.It is easier and more efficient to select among homogeneous progeny (versus heterogeneous progeny in conventional breeding).The cultivar released is homogeneous.
DisadvantagesThe procedure requires special skills and equipment in some cases.Additional technology for doubling may increase the cost of a breeding program.Frequency of haploids generated is not predictable.There is a lack of opportunity to observe line performance in early generations prior to homozygosity.
Unlike the conventional methods of inbreeding, it is possible to achieve completely homozygous individuals. Using an F 1hybrid or a segregating population as female parent in the production of maternally derived haploids increases genetic diversity in the doubled haploid line. It is advantageous if the female also has agronomically desirable traits. F 1hybrids are suitable because their female gametes will be segregating.
7.18 Germplasm preservation
Germplasm preservation in tissue culture was discussed in Chapter 6. This method of germplasm storage is often used for vegetatively propagated species.
Key references and suggested reading
1 Asif, M. (2013). Progress and Opportunities of Doubled Haploid Production, Springer briefs in plant science, vol. 6. Heidelberg: Springer.
2 Chaudhury, A.M., Ming, L., Miller, C. et al. (1997). Fertilization‐independent seed development in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America 94: 4223–4228.
3 Choo, T.M., Reinbergs, E., and Kasha, K.J. (1985). Use of haploids in breeding barley. Plant Breed Reviews 3: 219–252.
4 Forster, B.P., Heberle‐Bors, E., Kasha, K.J., and Touraev, A. (2007). The resurgence of haploids in higher plants. Trends in Plant Science 12: 368–375.
5 Hanna, W.W. and Bashaw, E.C. (1987). Apomixis: its identification and use in plant breeding. Crop Science 27: 1136–1139.
6 Humphreys, D.G. and Knox, R.E. (2015). Doubled Haploid Breeding in Cereals Advances in Plant Breeding Strategies: Breeding, Biotechnology and Molecular Tools, 241–290. New York: Springer.
7 Jain, M., Chengalrayan, K., Gallo‐Meacher, M., and Misley, P. (2005). Embryogenic callus induction and regeneration in a pentaploid hybrid bermudagrass. cv Tifton 85. Crop Science 45: 1069–1072.
8 Janhar, Elias, E.M., and Rao, M.B. (2004). Effects of growth regulators on in vitro plant regeneration in durum wheat. Crop Science 44: 1839–1846.
9 Kamo, K. (1995). A cultivar comparison of plant regeneration from suspension cells, callus, and cormel slices of Gladiolus. In Vitro Cellular and Developmental Biology 31: 113–115.
10 Kindinger, B., Bai, D., and Sokolov, V. (1996). Assignment of a gene (s) conferring apomixis in Tripsacum to a chromosome arm: cytological and molecular evidence. Genome 39: 1133–1141.
11 Koltunow, A.M., Bicknell, R.A., and Chaudhury, A.‐M. (1995). Apomixis: molecular strategies for the generation of genetically identical seeds without fertilization. Plant Physiology 108: 1345–1352.
Читать дальше













