In double chain silicates, an additional one-half bridging oxygen per tetrahedra joins two chains together. Minerals of this group are known as amphiboles , which occur widely in both igneous and metamorphic rocks. Among the important minerals in this group are hornblende (Ca 2Na(Mg,Fe) 4Al 3Si 8O 22(OH) 2), tremolite-actinolite (Ca 2(Mg,Fe) 5Si 8O 22(OH) 2), and glaucophane (Ca 2(Mg,Fe) 3Al 3Si 8O 22(OH) 2). These minerals all contain OH as an essential component (Cl or F sometimes substitutes for OH). They are thus examples of hydrous silicates.
Sharing of a third oxygen links the tetrahedra into sheets, forming the sheet silicates ( Figure 1.11d). This group includes micas such as biotite (K(Mg,Fe) 3AlSi 3O 10(OH) 2) and muscovite (KAl 3Si 3O 10(OH) 2), talc (Mg 3Si 4O 10(OH) 2), and clay minerals such as kaolinite (Al 2Si 2O 5(OH) 4). As in amphiboles, OH is an essential component of sheet silicates. Many of these minerals form through weathering and are thus primary sedimentary minerals. Many of them are found in igneous and metamorphic rocks as well.
When all four oxygens are shared between tetrahedra, the result is a framework. The simplest framework silicate is quartz (SiO 2), which consists solely of linked SiO 4tetrahedra. The other important group of framework silicates is the feldspars , of which there are three end-members: sanidine (KAlSi 3O 8), albite (NaAlSi 3O 8), and anorthite (CaAl 2Si 2O 8). The calcium and sodium feldspars form the plagioclase solid solution, which is stable through a large temperature range. Sodium and potassium feldspars, collectively called alkali feldspar, form more limited solid solutions, as we will find in Chapter 4. Feldspars are the most abundant minerals in the Earth's crust.
Because only a single anion or anionic group is present in the common minerals (the presence of OH –in many kinds of silicates is an important exception), minerals are classified compositionally by the nature of the anion or anionic group. Silicates, as we noted, are the most abundant compositional class. Other classes of minerals include oxides, in which one or more metals are bound to oxygen, for example, magnetite (Fe 3O 4) and ilmenite (FeTiO 3), Carbonates are a particularly important and abundant class of minerals at the Earth's surface, of which calcite (CaCO 3) is by far the most important. Other important groups include sulfates, such as gypsum (CaSO 4 .2H 2O), hydroxides such as gibbsite (Al(OH) 3), sulfides such as pyrite FeS 2, halides, such as halite (NaCl) and fluorite (CaF), and phosphates, of which only one, apatite (Ca 5(PO 4) 3(OH,F,Cl) is common. There are others as well, such as “native” minerals, consisting of a single element such as diamond, borates, arsenates, etc.
1.6 A BRIEF LOOK AT THE EARTH
1.6.1 Structure of the Earth
The Earth consists of three principal layers: the core, the mantle, and the crust ( Figure 1.12). The core, roughly 3400 km thick and extending about halfway to the surface, consists of Fe–Ni alloy and can be subdivided into an inner and outer core. The outer core is liquid while the inner core is solid. The mantle is nearly 3000 km thick and accounts for about two-thirds of the mass of the Earth, with the core accounting for the other third. The crust is quite thin by comparison, nowhere thicker than 100 km and usually much thinner. Its mass is only about 0.5% of the mass of the Earth. There are two fundamental kinds of crust: oceanic and continental. Ocean crust is thin (about 6 km) and, with the exception of the eastern Mediterranean, is nowhere older than about 180 million years. The continental crust is thicker (about 35–40 km thick on average) and relatively permanent, with an average age of ∼2 billion years.
Both the crust and the mantle consist principally of silicates. The mantle is comparatively rich in iron and magnesium, so ferromagnesian silicates, such as olivine and pyroxenes, dominate. Rocks having these compositional characteristics are called ultramafic . The continental crust is poor in iron and magnesium, and aluminosilicates such as feldspars dominate. Rocks of this composition are sometimes referred to as felsic (or silicic). The oceanic crust is intermediate in composition between the mantle and continental crust and has a mafic composition, consisting of a roughly 50:50 mix of ferromagnesian minerals and feldspar. These differences in composition lead to differences in density, which are ultimately responsible for the layering of the Earth: the density of the layers decreases outward. The continental crust is the least of dense of these layers. The fundamental reason why continents stick out above the oceans is that continental crust is less dense than oceanic crust. Not shown in the figure are two additional layers that are even less dense: the oceans or hydrosphere , which cover about two-thirds of the surface, and the atmosphere, which extends about 100 km above the surface.
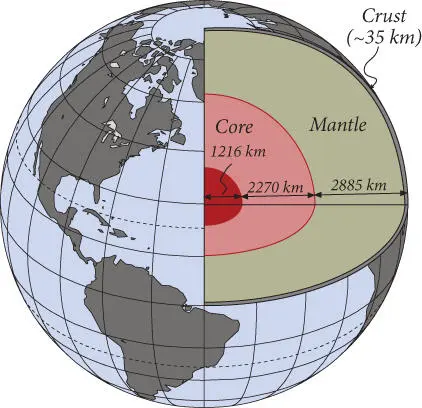
Figure 1.12 The Earth in cross-section. The outer rocky part of the planet, the mantle and crust, consists principally of silicates and is 2885 km thick. The core, divided into a liquid outer core and a solid inner core, consists of iron–nickel alloy and is 3486 km thick.
1.6.2 Plate tectonics and the hydrologic cycle
Two sources of energy drive all geologic processes: solar energy and the Earth's internal heat. Solar energy drives atmospheric and oceanic circulation, and with them, the hydrologic cycle. In the hydrologic cycle, water vapor in the atmosphere precipitates on the land as rain or snow, percolates into the soil and, through the action of gravity, makes its way to the oceans. From the oceans, it is evaporated into the atmosphere again and the cycle continues. The hydrologic cycle is responsible for two very important geologic processes: weathering and erosion. Weathering causes rocks to break down into small particles and dissolved components. The particles and dissolved matter are carried by the flow of water (and more rarely by wind and ice) from high elevation to areas of low elevation. Thus, the effect of the hydrologic cycle is to level the surface of the planet. From a geochemical perspective, it releases essential nutrients from rock that make life possible.
The Earth's internal heat is responsible for tectonic processes, which deform the surface of the planet, producing topographic highs and lows. The internal heat has two sources. Some fraction of the heat originated from the gravitational energy released when the Earth formed. The other fraction, 50% or less, of internal heat is produced by the decay of radioactive elements, principally uranium, thorium, and potassium, in the Earth. The Earth's internal heat is being slowly lost over geologic time as it migrates to the surface and is radiated away into space. It is this migration of heat out of the Earth that drives tectonic processes. Heat causes both the outer core and the mantle to convect, as hot regions rise and cold regions sink. Convection within the outer core gives rise to the Earth's magnetic field, and may have other, as yet not understood, geologic consequences. Convection in the mantle is responsible for deformation of the Earth's crust as well as volcanism. Solar energy drives convection in the oceans and atmosphere and is responsible for redistribution heat over the surface of the Earth and wind and ocean currents.
The great revolution in earth science in the 1960s centered on the realization that the outer part of the Earth was divided into a number of “plates” that moved relative to one another. Most tectonic processes, as well as most volcanism, occur at the boundaries between these plates. The outer part of the Earth, roughly the outer 100 km or so, is cool enough (<1000° C) that it is rigid. This rigid outer layer is known as the lithosphere and comprises both the crust and the outermost mantle ( Figure 1.13). The mantle below the lithosphere is hot enough (and under sufficient confining pressure) that it flows, albeit extremely slowly, when stressed. This part of the mantle is known as the asthenosphere. Temperature differences in the mantle create buoyancy stresses that produce convective flow. It is this flow that drives the motion of the lithospheric plates. The motion of the plates is extremely slow, a few tens of centimeters per year at most and generally much less. Nevertheless, on geologic time scales they are sufficient to continually reshape the surface of the Earth, creating the Atlantic Ocean, for example, in the last 200 million years and Himalayan Mountains in the last 40 million years.
Читать дальше













