1.5.4 Chemical bonding
1.5.4.1 Covalent, ionic, and metal bonds
Except for the noble gases, atoms rarely exist independently; they are generally bound to other atoms in molecules, crystals, or ionic radicals. Atoms bind to one another through transfer or sharing of electrons, or through electrostatic forces arising from uneven distribution of charge in atoms and molecules. A bond that results from the transfer of electrons from one atom to another is known as an ionic bond , an example is the bond between Na and Cl in a halite crystal. In this case, the Na atom (the electropositive element) gives up an electron, becoming positively charged, to the Cl atom (the electronegative element), which becomes negatively charged. Electrostatic forces between the Na +and the Cl –ions hold the ions in place in the crystal. When electrons are shared between atoms, such as in the H 2O or CH 4molecules or the 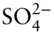 radical, the bond is known as covalent. In a covalent bond , the outer electrons of the atoms involved are in hybrid orbits that encompass both atoms.
radical, the bond is known as covalent. In a covalent bond , the outer electrons of the atoms involved are in hybrid orbits that encompass both atoms.
Ideal covalent and ionic bonds represent the extremes of a spectrum: most bonds are neither wholly covalent nor wholly ionic. In these intermediate cases, the bonding electrons will spend most, but not all, of their time associated with one atom or another. Electronegativity is useful in describing the degree of ionicity of a bond: a bond is considered ionic when the difference in the electronegativity of the two atoms involved is greater than 2. In Figure 1.5, we see that metals tend to have low electronegativities while the nonmetals have high electronegativities. Thus, bonds between metals and nonmetals (e.g., NaCl) will be ionic while those between nonmetals (e.g., CO 2) will be covalent, as will bonds between two like atoms (e.g., O 2).
Another type of bond occurs in pure metal and metal alloy solids. In the metallic bond, valence electrons are not associated with any single atom or pair of atoms; rather, they are mobile and may be found at any site in the crystal lattice. Since metals rarely occur naturally at the surface of the Earth (they do occur in meteorites and the Earth's core), this type of bond is less important in geochemistry than other bonds.
Ionically bonded compounds tend to be hard, brittle, and highly soluble in water. Covalently bonded compounds tend to be good conductors of heat, but not of electricity. They are typically harder and less brittle than ionic solids but less soluble. In molecular solids, such as ice, atoms within the molecule are covalently bonded. The molecules themselves, which occupy the lattice points of the crystal, are bonded to each other through van der Waals and/or hydrogen bonds. Such solids are comparatively weak and soft and generally have low melting points.
Molecules in which electrons are unequally shared have an asymmetric distribution of charge and are termed polar . A good example is the hydrogen chloride molecule. The difference in electronegativity between hydrogen and chlorine is 0.9, so we can predict that the bonding electron will be shared but associated more with the Cl atom than with the H atom in HCl. Thus, the H atom will have a partial positive charge, and the Cl atom a partial negative charge. Such a molecule is said to be a dipole . The dipole moment , which is the product of one of the charges (the two charges are equal and opposite) times the distance between the charges, is a measure of the asymmetric distribution of charge. Dipole moment is usually expressed in Debye units (1 Debye = 3.3356 × 10 –34coulomb-meters).
1.5.4.2 Van der Waals interactions and hydrogen bonds
Covalent and ionic bonds account for the majority of bonds between atoms in molecules and crystals. There are two other interactions that play a lesser role in interactions between atoms and molecules: van der Waals interactions and hydrogen bonds . These are much weaker but nevertheless play an important role in chemical interactions, particularly where water and organic substances are involved.
Van der Waals interactions arise from asymmetric distribution of charge in molecules and crystals. There are three sources for van der Waals interactions: dipole–dipole attraction , induction, and London dispersion forces. As we noted above, many molecules, including water, have permanent dipole moments. When two polar molecules encounter each other, they will behave much as two bar magnets: they will tend to orient themselves so that the positive part of one molecule is closest to the negative part of another ( Figure 1.7a). This results in a net attractive force between the two molecules. When the distance between molecules is large compared with the distance between charges within molecules, the energy of attraction can be shown to be:
(1.3) 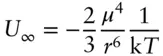
where U ∞is the interaction energy, μ is the dipole moment, T is temperature (absolute, or thermodynamic temperature, which we will introduce in the next chapter), k is a constant (Boltzmann's constant, which we shall also meet in the next chapter), and r is distance. We do not want to get lost in equations at this point; however, we can infer several important things about dipole–dipole interactions just from a quick glance at it. First, the interaction energy depends inversely on the sixth power of distance. Many important forces, such as electromagnetic and gravitational forces, depend on the inverse square of distance. Thus, we may infer that dipole−dipole forces become weaker with distance very rapidly. Indeed, they are likely to be negligible unless the molecules are very close. Second, the interaction energy depends on the fourth power of the dipole moment, so that small differences in dipole moment will result in large differences in interaction energy. For example, the dipole moment of water (1.84 Debyes) is less than twice that of HCl (1.03 Debyes), yet the dipole interaction energy between two water molecules (716 J/mol) is nearly 10 times as great as that between two HCl molecules (72.24 J/mol) at the same temperature and distance (298 K and 50 pm). Finally, we see that dipole interaction energy will decrease with temperature.
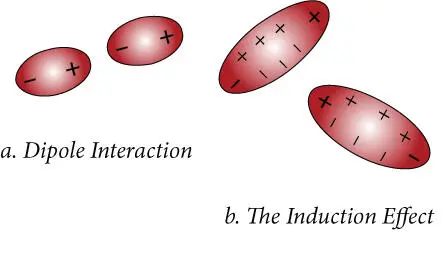
Figure 1.7 Van der Waals interactions arise because of the polar nature of some molecules. Illustrated here are (a) dipole−dipole interactions, which occur when two dipolar molecules orient themselves so oppositely charged sides are closest, and (b) the induction effect, which arises when the electron orbits of one molecule are perturbed by the electromagnetic field of another molecule.
Dipole molecules may also polarize electrons in a neighboring molecule and distort their orbits in such a way that their interaction with the dipole of the first molecule is attractive. This is known as the induction effect ( Figure 1.7b). The induction energy also depends on the inverse sixth power of intermolecular distance, but only on the square of the dipole moment of the molecules involved. In addition, another parameter, the polarizability of a molecule, is also needed to describe this effect. In general, the attraction arising from induction is less important than from dipole–dipole interaction. However, because it depends only on the square of dipole moment, the induction attraction can be larger than the dipole–dipole attraction for some weakly dipolar molecules.
Читать дальше

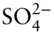 radical, the bond is known as covalent. In a covalent bond , the outer electrons of the atoms involved are in hybrid orbits that encompass both atoms.
radical, the bond is known as covalent. In a covalent bond , the outer electrons of the atoms involved are in hybrid orbits that encompass both atoms.