Finally, van der Waals forces can also occur as a consequence of fluctuations of charge distribution on molecules that occur on time scales of 10 –16seconds. These are known as London dispersion forces. They arise when the instantaneous dipole of one molecule induces a dipole in a neighboring molecule. As was the case in induction, the molecules will orient themselves so that the net forces between them are attractive.
The total energy of all three types of van der Waals interactions between water molecules is about 380 J/mol, assuming an intermolecular distance of 5 pm and a temperature of 298 K (25°C). Though some interaction energies can be much stronger (e.g., CCl 4, 2.8 kJ/mol) or weaker (1 J/mol for He), an energy of a few hundred joules per mole is typical of many substances. By comparison, the hydrogen–oxygen bond energy for each H–O bond in the water molecule is 46.5 kJ/mol. Thus, van der Waals interactions are quite weak compared with typical intramolecular bond energies.
The hydrogen bond is similar to van der Waals interactions in that it arises from nonsymmetric distribution of charge in molecules. However, it differs from van der Waals interactions in a number of ways. First, it occurs exclusively between hydrogen and strongly electronegative atoms, namely oxygen, nitrogen, and fluorine. Second, it can be several orders of magnitude stronger than van der Waals interactions, though still weak by comparison with covalent and ionic bonds. In the water molecule, binding between oxygen and hydrogen results in hybridization of s and p orbitals to yield two bonding orbitals between the O and two H atoms, and two nonbinding sp 3orbitals on the oxygen. The latter are prominent on the opposite side of the O from the hydrogens. The hydrogen in one water molecule, carrying a net positive charge, is attracted by the nonbinding sp 3electrons of the oxygen of another water molecule, forming a hydrogen bond with it ( Figure 1.8).
Hydrogen bonds typically have energies in the range of 20–40 kJ/mol. These are much higher than expected for electrostatic interactions alone, and indeed approach values similar to intramolecular bond energies. Thus, there is the suspicion that some degree of covalency is also involved in the hydrogen bond. That is to say, the nonbinding electrons of oxygen are to some degree shared with the hydrogen in another molecule. Hydrogen bonds are perhaps most important in water, where they account for some of the extremely usual properties of this compound, such as its high heat of vaporization, but they can also be important in organic molecules and are present in HF and ammonia as well.
1.5.5 Molecules, crystals, and minerals
1.5.5.1 Molecules
Molecules, which result from the chemical bonds between atoms discussed above, are a familiar concept. By some definitions, they are electrically neutral; charged species consisting of two or more atoms are known as radicals . The properties of molecules are generally quite different from those of their constituent atoms. Carbon dioxide is a good example: the equilibrium form of pure carbon at the room temperature and pressure is a solid, graphite, while that of oxygen is a diatomic gas. The properties of molecules depend on the bond lengths, which depend on the strength of the chemical bond, as well as the geometric arrangement of the atoms. For example, the polar nature of the water molecule, from which many of its unusual properties arise (and which will discuss in Chapter 3), including the formation of hydrogen bonds, is a consequence of the tendency of valence electron pairs surrounding an atom tend to repel each other which results in an arrangement in which the two hydrogens are separated by an angle of 104.45° ( Figure 1.8a) and a partial positive charge on the hydrogens and a partial negative one on the other side of the molecule In contrast, the arrangement of atoms in CO 2is linear with a bond angle of 180°. CO 2reacts with water to produce carbonic acid (H 2CO 3), which has a plane trigonal geometry.
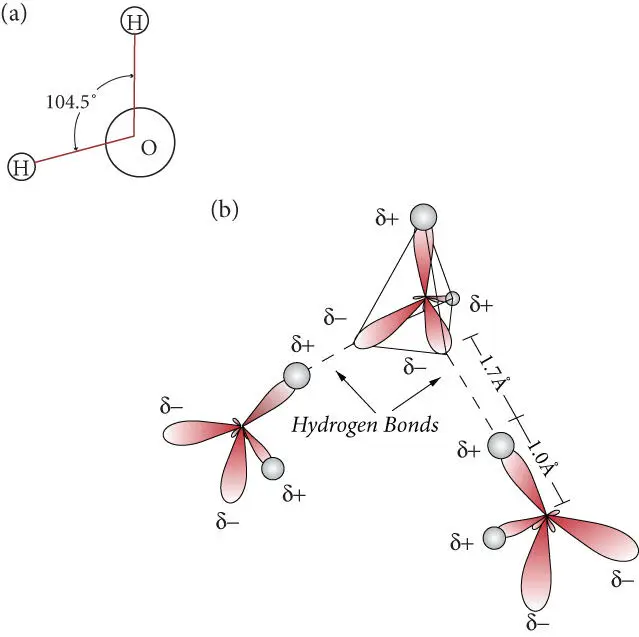
Figure 1.8 (a) Geometry of the water molecule. (b) Hydrogen bonds between water molecules. The δ+ and δ– indicate partial positive and negative charges, respectively.
Geometry becomes enormously important for organic molecules and life. For example, C 12H 22O 11is the chemical formula for both lactose and sucrose, as well as several other disaccharide carbohydrates, but the atoms are stitched together differently and as a result they have quite different properties. Among other things, all adults (and essentially all animals) can readily digest sucrose, but many adult humans (and most adult mammals) cannot digest lactose. In other molecules, even slight variations in structure, for example, a molecular structure and its mirror image can have quite different properties – a topic we'll explore briefly in Chapter 12.
Molecules are not necessarily static entities. An important feature of some molecules is the ability to dissociate. This is particularly true of both water and carbonic acid, which can give up hydrogen atoms. Acidity reflects the balance between H +(strictly speaking H 3O +) and OH –ions; these must be equal in pure water, but a solution of CO 2in water will have an excess of H +and hence be acidic. These hydrogen ions can also reassociate with their parent molecules and do so when H ions become abundant.
The bonds between atoms in molecules are also not static, but rather bond lengths and bond angles continually oscillate about their mean values. For example, the water molecule has three fundamental modes of vibration: two stretching vibrations of the O-H bond (one symmetric, one asymmetric) and a bending vibration in which the bond angle changes. Vibrational frequencies are proportional to bond strength and increase with increasing temperature (in stepwise fashion, as they are quantized), although many molecules remain in the ground state vibrational frequencies over the range of temperatures at the Earth's surface. These vibrations are responsible for some of the interaction of molecules with light. For example, the frequencies of the stretching vibrations of water and CO 2correspond to near-infrared electromagnetic frequencies and as a consequence both molecules absorb infrared photons, and hence both are important greenhouse gases.
The solid Earth, however, is not made up of molecules. Instead, it is made up almost entirely of minerals. By definition, a mineral is a crystalline solid. Crystals are infinitely repeating lattice structures that define the fixed positions of atoms and the geometric relationships between them (but just as in molecules, atoms vibrate in crystals). Just as the chemical formula of molecule, for example C 12H 22O 11, does not tell us all we need to know about that compound, a chemical formula of a crystal such as quartz, SiO 2, does not tell us everything we need to know about that crystal as SIO 2has several polymorphs, such as cristobalite and tridymite. Nothing demonstrates this better than the difference between the two polymorphs of carbon: graphite and diamond, which differ only in the way carbon atoms are bound together.
The most abundant elements in the Earth are O and Fe (both close to 32%), Mg (∼15%), Si (∼14%), Ni (∼1.8%), Ca (1.7%), and Al (1.6%). The majority of the Earth's Fe and Ni are in the core. The remaining rocky part of the Earth, the mantle and crust, consists of ∼44% O, ∼23% Mg, ∼21% Si, ∼8% Fe, ∼2.5% Ca, and ∼2.4% Al. As a consequence, the outer part of the Earth consists almost entirely of silicate minerals, in which the basic structural unit is 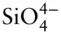 , in which four oxygens are arranged around the silicon atom to form a tetrahedron ( Figure 1.9a). The simplest of silicates is quartz, with a chemical formula of SiO 2.
, in which four oxygens are arranged around the silicon atom to form a tetrahedron ( Figure 1.9a). The simplest of silicates is quartz, with a chemical formula of SiO 2.
Читать дальше


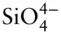 , in which four oxygens are arranged around the silicon atom to form a tetrahedron ( Figure 1.9a). The simplest of silicates is quartz, with a chemical formula of SiO 2.
, in which four oxygens are arranged around the silicon atom to form a tetrahedron ( Figure 1.9a). The simplest of silicates is quartz, with a chemical formula of SiO 2.










