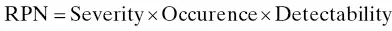2.5. QbD APPROACH TO OPTIMIZE EMULSION
The QbD paradigm, as clearly delineated in the International Council for Harmonisation (ICH) Q8(R2), Q9, and Q10 guidelines, indicates the necessity to follow initial risk assessment studies, factor screening studies, and then the optimization of any pharmaceutical products not only for improving the final product quality but also to provide regulatory flexibility for the industry to improve their manufacturing processes [ICH Q9 guideline 2005; ICH Q10 guideline 2008; ICH Q8(R2) guideline 2009]. The guideline ICH Q8(R2) described a QbD‐based approach of Formulation by Design (FbD). Whereas the ICH Q9 promulgated the need of Quality Risk Management (QRM), the ICH Q10 signified the way of obtaining the quality final products. The typical steps required for the new API products during their formulation development stage are shown in Flowchart 2.1.
Like other dosage forms, the o/w nanosized emulsions do contain many raw materials, manufacturing process, and testing of in‐process materials and final products. At the very outset, the selection of each raw materials and their proper amount is one of the prime steps. Following the raw materials’ assortment, the manufacturing process is to be selected from the myriad of available size‐reduction machineries. In many cases, these two (raw materials and manufacturing process) constraints are often called as independent variables or critical process parameters (CPPs). Studying the influence of CPPs on the so‐called dependent or response variables [also known as critical quality attributes (CQAs)] will eventually determine the final product quality and also provide flexibility for the industry to advance their manufacturing processes for meeting the stringent regulatory procedures. Hence, the studies relating the influence of CPPs on CQAs look in a diverse manner that depends on the changes of regulatory procedures over the time periods. Initially, it was thought that the product quality along with its performance, in vitro and in vivo , could be safeguarded by performing the traditional quality by testing (QbT) approach usually via one‐factor‐at‐a‐time (OFAT) experimental approach. In the QbT approach, the quality of both API and its product is mainly ensured by testing of raw materials, a fixed manufacturing process, and testing of in‐process materials and end product (Yu 2008).
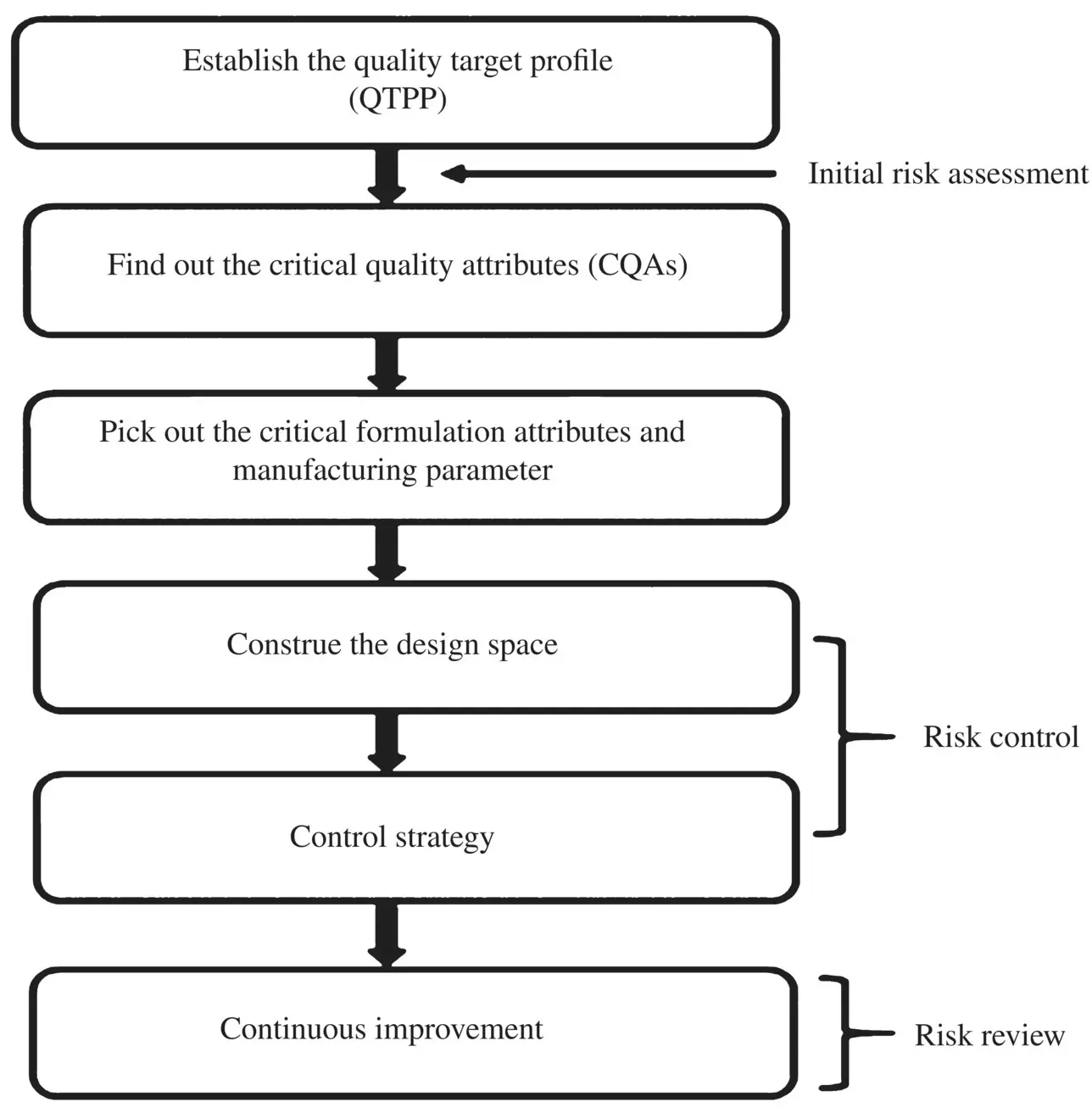
Flowchart 2.1. Typical steps involved for the new drug products during their formulation development stage as per the quality by design (QbD) approach of formulation by design (FbD).
This traditional framework has certain drawbacks. Any minor changes made in input materials and processes (including equipment) for anticipated variability are empirical and addressed via the OFAT experimental approach. This development practice is not cost‐effective and results in incomplete product and process understandings, which in turn leads to restrictive (or fixed) manufacturing processes that are unable to compensate for the regular variability in input materials, processes, manufacturing equipment, and laboratory instrumentation (Debevec et al. 2018). As mentioned earlier, the QbT approach also requires extensive testing to comply with restrictive FDA‐approved specifications (Yu 2008).
For these reasons, traditional industry practices are often extraordinarily expensive and time consuming and present an overwhelming burden to regulatory agencies for reviewing multiple chemistry, manufacturing, and control (CMC) supplements. Another major drawback of the traditional approach is that it does not differentiate products with regard to complexity (for example, APIs with high potency and/or narrow therapeutic indices) and associated level of quality and health risks to manufacturers and consumers, respectively (Yu 2008). These drawbacks of “minimal” or “traditional approach” and need for higher regulatory flexibility via an “enhanced approach” prompted a paradigm shift in the industry practice [ICH Q8(R2) guideline 2009].
The need for transition from traditional QbT to an enhanced approach was formally communicated through an ICH Q8 guidance published in May 2006, which emphasized that “quality cannot be tested into products, rather it should be built into products by design” (FDA Guidance for Industry 2006).
These innovative frameworks are fully reflected in current regulatory guidance on QbD and PAT [FDA Guidance for Industry 2004; ICH Q8(R2) guideline 2009] and are encouraged for industry practice.
Both QbD and PAT share common goals of providing a rapid and science‐ and risk‐based road map for product development and economically effective strategies for process monitoring and analytical testing. The QbD strategy involves an end‐to‐end integration of six key elements, which are quality target product profile (QTPP), risk assessments related to process and product design, DOEs, design space, control strategy, and continuous process improvement. Each of these elements is essential for product development, manufacturing, and quality assurance. Through parallel evolution of QbD and PAT, it is now well established that developing a robust formulation and manufacturing process requires a thorough understanding of inter‐relationships between material attributes (MAs), processing parameters (PPs), and dependent product quality attributes at each stage of product development. In this regard, pharmaceutical, chemical, and engineering industries, research institutions, and regulatory agencies worldwide have significantly contributed through joint collaborations, workshops, conference presentations, and publications. More recently, a 5‐year pilot QbD program (March 2011–April 2016) was launched between the FDA and the EMEA (EMA 2017).
In this pilot program, the subject matter experts from both agencies had thoroughly exchanged their viewpoints to allow a joint evaluation of QbD elements in an effort to harmonize agencies’ expectation for regulatory submissions worldwide. As a result of this pilot program, both the FDA and the EMEA have mutually agreed on several pertinent topics and level of details required in a regulatory submission (EMA 2014).
These include risk assessments related to process and product design, DOEs, and design space, which are briefly summarized below. In QbD‐based product development, the MAs and PPs are initially identified from prior scientific knowledge using risk assessment techniques. One of commonly utilized risk assessment tools is failure mode effect analysis (FMEA). In this approach, a risk priority number (RPN) is calculated for each factor that can impact the CQAs of the product. The RPN is calculated by multiplying numerical rankings (e.g., 1–5 or 1–10) ascribed to three risk components (severity of harm, probability of occurrence, and detectability) associated with each factor, i.e.,
(2.1) 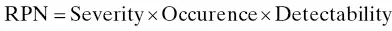
The RPN values can be presented in a tabular format or as a Pareto plot for a quantitative display of relative risk rank order. It needs to be emphasized that all the MAs and PPs that can potentially affect CQAs are considered as part of the initial risk analysis; however, only a subset of these attributes and parameters are selected for development studies as warranted by the outcome of risk analysis (Badawy et al. 2016).
This QbD element has originated from the Pareto principle. As a rule of thumb, about 80% of the problems originate from roughly 20% of the factors identified, that is why Pareto concept is sometimes referred to as 80/20 rule (Orloff 2011).
Читать дальше