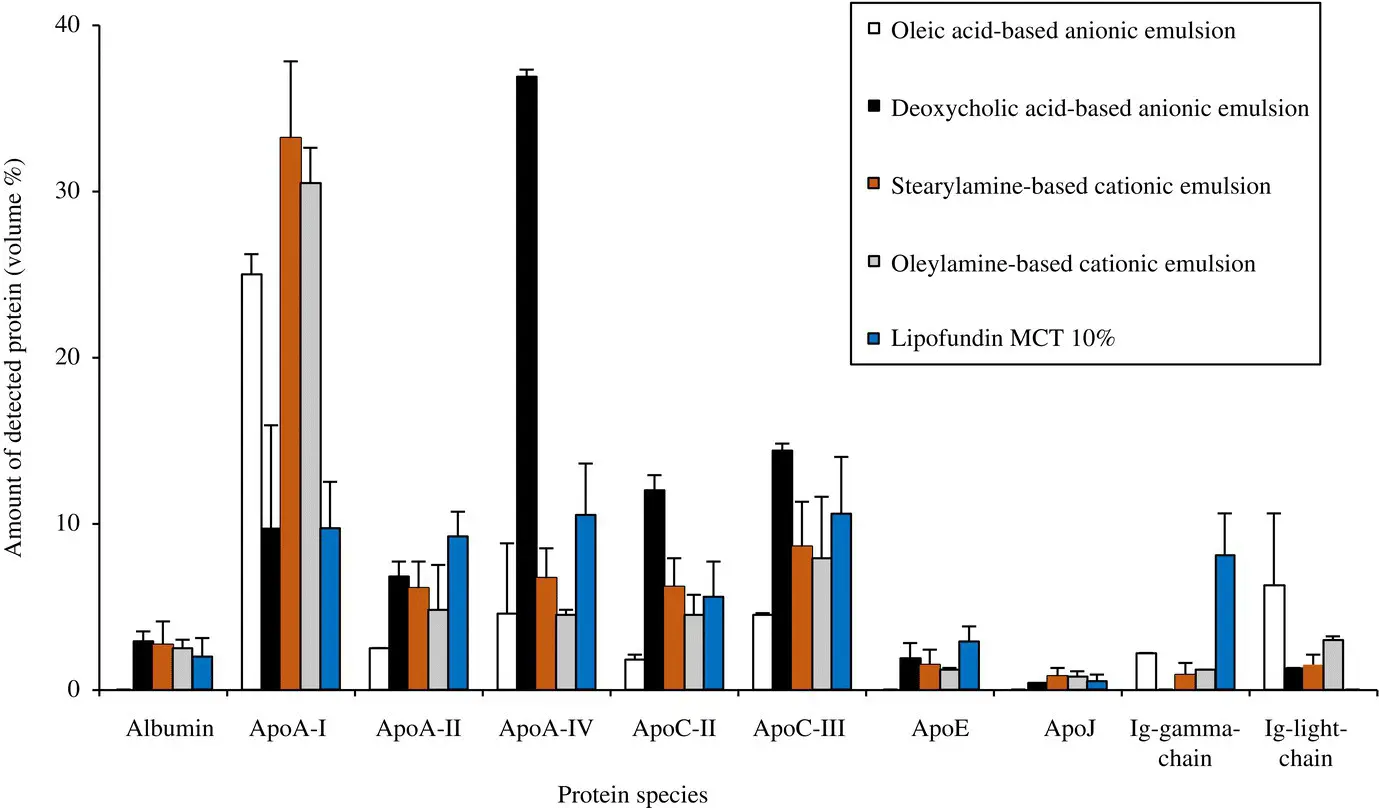
Figure 2.2. Amount of major proteins on the 2‐D gels of plasma proteins adsorbed on emulsions with negative or positive surface charge in comparison with Lipofundin MCT 10%.
[Taken with permission from Elsevier (Tamilvanan et al. 2005).]
2.2.7. Advantages of Stabilizers in Nanosized Emulsions
In some practical cases where emulsions are applied under real, sometimes quite harsh conditions, such as high temperature or shear stresses, the stabilization of colloidal carriers by conventional surfactants (ionic as well as nonionic) can appear to be insufficient to keep the initially acceptable emulsion properties intact. For ionic surfactants as stabilizers, the stabilization mechanism based on the electrical double layer fails at high amounts of electrolyte. The situation for nonionic surfactants as stabilizers is only slightly better because their molecules are typically not strongly adsorbed (Tadros et al. 2004; Tadros 2006). Therefore, the most effective way to stabilize emulsions by creation of a protective adsorption layer is the use of amphiphilic macromolecular compounds or polymeric surfactants (Tadros et al. 2004). In contrast to the commonly used monomolecular surfactants, polymeric surfactants do not only adsorb much stronger but also retain this ability under high electrolyte concentration or/and high temperature (Tadros et al. 2004; Tadros 2006). This is made possible due to a special molecular design of surface active polymers that have in their structure anchor groups responsible for strong adsorption at the interface and stabilizing groups protruding from the interface into the dispersion medium and forming a bulky layer with thicknesses of several nanometers.
Most often used stabilizers for the preparation of emulsions, in the fields of agrochemicals, pharmaceuticals, and personal care products, are either block or graft copolymers. In block copolymers, the hydrophobic blocks reside at the surface or even partly penetrate in the oil droplet, making trains or short loops whereas the hydrophilic blocks protrude in the dispersion medium as loops or tails providing steric stabilization (Benichou et al. 2004). As examples, PEO‐PPO‐PEO triblock copolymer (commercially available as “Pluronics”) or PPO‐PEO‐PPO can be mentioned. Triblock copolymers are, however, not the most efficient stabilizers because the PPO chain is not hydrophobic enough to attach strongly at the o/w interface (Benichou et al. 2004). The surface activity of these polymeric surfactants is rather the result of a rejective anchoring or negative enthalpic energy change of the PPO group because of its low solubility in water and most oils. Alternative and more efficient graft copolymers consist of a polymeric backbone attached to the interface and several chains dangling into the continuous phase and forming at the interface a “brush” structure.
A typical example of commercial graft was described (Jumaa and Müller 2002). Here, mixtures of polyoxyethylene‐660‐12‐hydroxystearate (Solutol HS15) with the anionic lipid composition. Lipoid S75 was employed to enhance the long term as well as accelerated (by freezing and centrifugation) stability of o/w nanosized emulsions. Emulsion stabilized by phospholipids displayed a stable behavior after autoclaving and centrifugation but de‐emulsified after freezing. In contrast, emulsions prepared only with Solutol HS15 demonstrated a significant change in particle size after autoclaving. The best results were obtained using a stabilizer mixture revealing a combination of electrostatic stabilization mechanism typical for the anionic phospholipids and the steric stabilization mechanism originating from nonionic polymeric surfactant. The combination of stabilization mechanisms improved the emulsion's stability, compared to the emulsion's stability prepared using only the individual surfactants.
The commercially available cationic block‐copolymer Eudragit E100 was utilized as both emulsion stabilizer and solidifying agent upon further drying (Cui et al. 2007). Due to the specific properties of Eudragit E100, no surfactant or organic solvent additives were employed in order to fulfill common ecological, toxicological, and manufacturing safety requirements during the preparation of a redispersible dry emulsion.
The naturally occurring polymers cyclodextrins are commonly used in pharmaceutical aqueous formulations for inclusion of less soluble or instable APIs. Their use, however, is complicated by poor interaction with biological membranes. To overcome this difficulty, the cyclodextrins were chemically modified, including grafting of fatty acid chains to the hydroxyl groups in order to provide the optimum surface activity to the polymer (Memisoglu et al. 2002). Upon addition of Miglyol to an aqueous phase, nanocapsules with an oily interior were formed spontaneously. These nanocapsules had an average size of approximately 300 nm and revealed an excellent long‐term stability for at least 5 months. Interestingly, the most appropriate properties for nanocapsule formation were observed for cyclodextrin derivatives with linear C 6chains. Compounds possessing longer aliphatic chains caused the formation of large polydisperse aggregates and were therefore unsuitable for encapsulation. The preparation of nanocapsules with modified cyclodextrins without the addition of surfactants is a promising tool for the development of novel API delivery systems. A similar approach of adding cyclodextrin instead of surfactant molecules to stabilize the o/w nanosized emulsions is currently receiving much attention (as briefly discussed in Chapter 7). Furthermore, a variety of current approaches to increase emulsion stability with help of emulsifier molecules have been summarized in a recent review by Grigoriev and Miller (2009).
2.2.8. Miscellaneous Additives
Additives other than antioxidants such as preservatives (e.g., benzalkonium chloride, chlorocresol, and parabens) are included in emulsions to prevent microbial spoilage of multidose medical emulsions. α ‐Tocopherol is a good example of an antioxidant used to obtain a desirable stabilized emulsion under prolonged storage conditions. The presence of components of natural origin such as lecithin or oils with high calorific potential renders the emulsion a good medium to promote microbial growth when it is packaged in multidose containers. Pharmaceutical products when distributed into multidose containers, especially for parenteral and ocular administrations, should be properly preserved against microbial contamination and proliferation during storage under normal conditions and proper use. Incorporation of preservatives in single‐dose vials is also a common procedure if filtration is used as a sterilization method (Tamilvanan 2008). Sznitowska et al. (2002) studied the physicochemical compatibility between the lecithin‐stabilized emulsion and 12 antimicrobial agents over 2 years of storage at room temperature. Preliminary physicochemical screening results indicated that the addition of chlorocresol, phenol, benzyl alcohol, thiomersal, chlorhexidine gluconate, and bronopol should be avoided due to the occurrence of an unfavorable pH change followed by coalescence of lecithin‐stabilized droplets of the emulsion.
Furthermore, the efficacy of antimicrobial preservation was assessed using the challenge test according to the method described by the European Pharmacopoeia.
Despite good physicochemical compatibility, neither parabens nor benzalkonium chloride showed satisfactory antibacterial efficacy in the emulsion against the tested microorganisms and consequently were not suitable for preservation. Therefore, higher concentrations of antimicrobial agents or their combinations may be required for efficient preservation of the lecithin‐stabilized emulsion probably because of unfavorable phase partitioning of the added antimicrobials within the different internal structures of the emulsion (Sznitowska et al. 2002). This finding clearly indicates that the possible electrostatic attraction between the negatively charged lipid moieties of the mixed emulsifying film formed around the anionic emulsified oil droplets (Abele et al. 1997) and the quaternary cationic ammonium groups of the preservative is not the plausible cause for the reduced activity of the benzalkonium chloride. Thus, the possible intercalation of this surfactant in either the cationic or anionic interfacial mixed emulsifying film is likely to occur, preventing benzalkonium chloride from eliciting its adequate preservative action (Sznitowska et al. 2002).
Читать дальше













