an interfacial incorporation approach, which includes the recently developed SolEmul® technology, and
incorporation of antibodies, DNA protein, oligonucleotide, or heat labile molecules.
2.4.1. Extemporaneous API Addition
Cohen et al. (1996), when looking for a new galenic presentation form for amphotericin B with better ocular tolerance over the commercially available Fungizone ®eye drops, incorporated the API directly into the preformed 20% emulsion, Intralipid ®. However, after addition of the solid API particles or API solution, several physical changes such as phase separation, precipitation, or creaming may occur thus limiting such practices in o/w nanosized emulsion preparations. Therefore, ocular active lipophilic agents are not normally incorporated into the emulsions by this extemporaneous addition method.
2.4.2. De Novo Emulsion Preparation
In principle, the lipophilic API molecules (thermostable) should however be incorporated by a de novo process as described earlier. Thus, the API is initially solubilized or dispersed together with an emulsifier in suitable single‐oil or oil mixture by means of heating. The water phase containing the osmotic agent with or without an additional emulsifier is also heated and mixed with the oil phase by means of high‐speed mixers. Further homogenization takes place to obtain the needed small droplet size range of the emulsion. A terminal sterilization by filtration, or steam, then follows. The emulsion thus formed contains most of the API molecules within its oil phase or its oil–water interface. This is a generally accepted and standard method to prepare lipophilic API‐loaded nanosized emulsions for parenteral, ocular, percutaneous, and nasal uses, as illustrated in Fig. 2.3. This process is normally carried out under aseptic conditions and nitrogen or argon atmosphere to prevent both contamination and potential oxidation of sensitive excipients.
2.4.3. Interfacial Incorporation Approach
Since many APIs of commercial interest generally have a solubility that is too low in FDA‐approved oils, Lance et al. (1995) proposed a method to incorporate such APIs into the interfacial o/w layer of the emulsion droplets. This can be achieved by initially dissolving the API along with the phospholipid (emulsifier) in an organic solvent, instead of in the oil.
Following the solvent evaporation, the obtained phospholipids/API co‐mixture is used in the de novo production of the emulsions (Davis and Washington 1988). However, this approach suffers from possible API nanocrystal formation and from the use of organic solvent during the emulsion preparation process. To overcome such drawbacks, a novel SolEmul ®technology was developed in which an additional high‐speed homogenization step is included to mix the API with emulsion. The API particles are micronized to the nanosize range prior to incorporation into the emulsions. By this technique, adequate amounts of lipophilic APIs can be substantially incorporated into the lipophilic core or intercalated between the selected emulsifier molecular films at the o/w interface of the emulsions. The APIs reported to have been incorporated by this novel approach are amphotericin B, carbamazepine, and itraconazole (Buttle et al. 2002; Müller and Schmidt 2002; Akkar and Müller 2003a, b). However, it should be emphasized that all the lipophilic API molecules that have been incorporated into the emulsions by SolEmul ®technology are meant only for parenteral use (Buttle et al. 2002; Müller and Schmidt 2002; Akkar and Müller 2003a, b) and so far no ocular, nasal, and topical active agents have been incorporated by this approach although there is no regulatory reason to exclude this technical improvement when designing emulsion formulations for these applications.
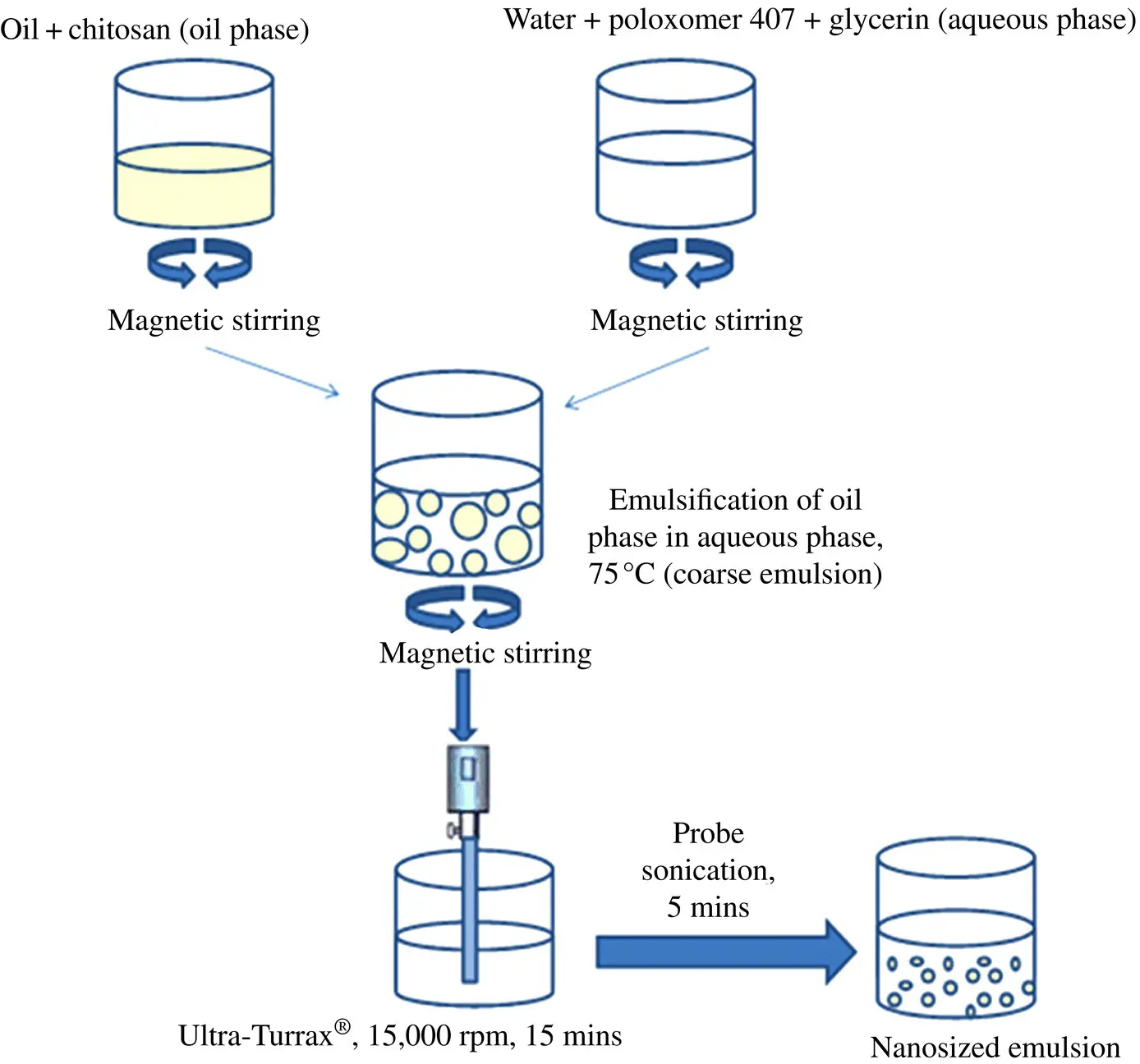
Figure 2.3. Schematic diagram for preparation of nanosized emulsion.
2.4.4. Incorporation of Antibodies, DNA Protein, Oligonucleotide, or Heat Labile Molecules
Both extemporaneous API addition (method A) into the preformed emulsion and de novo emulsion preparation (method B) are useful for the incorporation of heat labile molecules into the o/w nanosized emulsions. For example, cyclosporin A (peptidic molecule) was successfully incorporated without API degradation into the emulsion by following the de novo method (Tamilvanan et al. 2001). The extemporaneous addition of the solid API or API previously solubilized in another solvent or oil to the o/w nanosized emulsions is not a favored approach in technology wise as it might compromise the integrity of the emulsion. However, since therapeutic DNA and single‐stranded oligos or siRNA are water soluble due to their polyanionic character, the aqueous solution of these compounds need to be added directly to the o/w cationic nanosized emulsion in order to interact electrostatically with the cationic emulsion droplets and thus associate/link superficially at the oil–water interface of the emulsion (Teixeira et al. 1999; Tamilvanan 2004; Hagigit et al. 2010). During in vivo condition when administered via parenteral and ocular routes, the release of the DNA and oligos from the associated emulsion droplet surfaces should therefore initially be dependent solely on the affinity between the physiological anions of the biological fluid and cationic surface of the emulsion droplets. The mono‐ and di‐valent anions containing biological fluid available in parenteral route is plasma and in ocular topical route is tear fluid, aqueous humor, and vitreous. Moreover, these biofluids contain multitude of macromolecules and nucleases. There is a possibility that endogenous negatively charged biofluid's components could dissociate the DNA and oligos from cationic emulsion. It is noteworthy to conduct during the preformulation development stages an in vitro release study for therapeutic DNA and oligos‐containing cationic nanoemulsion in these biological fluids and this type of study could be considered as an indicator for the strength of the interaction occurred between DNA or oligo and the emulsion (Hagigit et al. 2008). Interestingly, the stability of oligos (a 17‐base oligonucleotide, partially phosphorothioated) was validated using a gel‐electrophoresis method. After incorporating the oligos into the cationic nanosized emulsion as well as during in vitro experiments of oligos‐containing emulsion in vitreous fluid at different time periods, the emulsions were phase separated by Triton X‐100 and then the degradation of oligos was also monitored following the same validated gel‐electrophoresis method (Hagigit et al. 2008). No appearance of new band was seen in comparison to the standard aqueous oligos solution. This result indicates that the oligos did not undergo degradation against the conditions applied to prepare a sterile emulsion.
In order to bring the nanosized emulsion closer to otherwise inaccessible pathological target tissues, homing devices/ligands such as antibodies and cell recognition proteins are usually linked somehow onto the particle surfaces. Various methods have been employed to couple ligands to the surface of the nanosized emulsions with reactive groups. These can be divided into covalent and noncovalent couplings. Noncovalent binding by simple physical association of targeting ligands to the nanocarrier surface has the advantage of eliminating the use of rigorous, destructive reaction agents. Common covalent coupling methods involve formation of a disulfide bond, cross‐linking between two primary amines, reaction between a carboxylic acid group and primary amine, reaction between maleimide and thiol, reaction between hydrazide and aldehyde, and reaction between a primary amine and free aldehyde (Nobs et al. 2004). For antibody‐conjugated anionic emulsions, the reaction of the carboxyl derivative of the coemulsifier molecule with free amine groups of the antibody and disulfide bond formation between coemulsifier derivative and reduced antibody were the two reported conjugation techniques so far (Song et al. 1996; Lundberg et al. 1999, 2004). However, by the formation of a thio‐ether bond between the free maleimide reactive group already localized at the o/w interface of the emulsion oil droplets and a reduced monoclonal antibody, the antibody‐tethered cationic emulsion was developed for active targeting to tumor cells (Goldstein et al. 2005, 2007a, b).
Читать дальше













