Einstein’s success in producing such a mathematical model that replicated the motion of the pollen was a devastating blow to anyone who believed that a liquid like water was a continuous substance. It was very hard for anyone who still believed in Aristotle’s view of matter to come up with a comparably convincing explanation.
The calculations allowed one to estimate how small the molecules of water were in comparison with the pollen they were knocking around. Although it was convincing evidence that matter came in discrete pieces, it did not answer the question of whether you could still infinitely divide these pieces into ever smaller parts.
Indeed, the indivisible atoms turned out to be far from indivisible with the discovery of smaller constituents that made up atoms of carbon or oxygen. The next layer down revealed that an atom is made up of even tinier pucks called electrons, protons and neutrons, the first of which had already come to light some eight years before Einstein’s theoretical breakthrough.
The way science works is that you can hang on to your model of the universe until something pops up that doesn’t fit: something new that you can’t seem to explain with the current model. The realization that the atom might be made up of smaller bits emerged out of experiments that revealed something particle-like but much tinier than the atoms that made up the periodic table.
This tiny particle-like object materialized from the British physicist J. J. Thomson’s experiments at the end of the nineteenth century to understand electricity. He had been investigating how electricity was conducted through a gas. Early experiments took a glass tube with two electrodes at either end, and by applying a high voltage between the electrodes an electric current was produced. The strange thing was that he seemed to be able to actually see the current because an arc of light appeared between the two electrodes.
Things became even stranger when he removed the gas completely from the tube and applied the voltage across a vacuum. The arc of light disappeared. But, bizarrely, the glass at the end of one tube was found to fluoresce. Stick a metal cross in the tube and a cross-shaped shadow appeared in the middle of the glowing fluorescent patch.
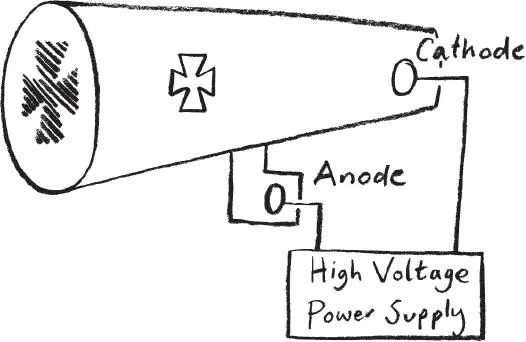
Electrons emitted from the cathode that hit the opposite wall cause the glass to fluoresce.
The shadow always appeared opposite the negative electrode, otherwise known as the cathode. The best explanation was that the cathode was emitting some sort of ray that interacted with matter and made it glow – either the gas in the tube or, in the case of the vacuum, the glass of the tube itself.
These ‘cathode rays’ were something of a mystery. They were found to pass right through thin sheets of gold when they were placed in the way. Were they some sort of wave-like phenomenon like light? Others thought they were made up of negatively charged particles spat out by the negative electrode and then attracted to the positive electrode. But how could these particles pass through solid gold?
If they were negatively charged particles, then, Thomson believed, he should be able to change their path through the tube by applying a magnetic field. The German physicist Heinrich Hertz had already tried this and failed, but Hertz hadn’t removed enough gas, which interfered with the experiment. With the gas removed, things worked just as Thomson had hoped. Apply a magnetic field to the rays and sure enough the shadow shifted. The rays were being bent by the magnet.
The real surprise came when Thomson made a mathematical calculation of what the mass of these charged particles must be. If you apply a force to a mass then, as Newton’s laws of motion state, the amount you’ll be able to move it will depend on the mass. So the amount of deflection that a magnetic field will cause will have encoded in it information about the mass of this proposed particle.
The calculation also depends on the charge on the particle, and once this was determined in a separate experiment Thomson could work out the mass. The answer was startling. It was nearly 2000 times smaller than the mass of a hydrogen atom, the smallest atom in the periodic table.
That these particles seemed to originate from the metal making up the electrode led to the hunch that these particles were actually smaller constituents of the atom. The atom wasn’t indivisible after all. There were smaller bits. They were called electrons, the name originating from the Greek word for amber, the first substance to exhibit a charge.
The discovery that atoms are made up of even smaller constituents was a shock to many scientists’ view of the world. After Thomson gave a lecture on his findings:
I was told long afterwards by a distinguished physicist who had been present at my lecture that he thought I had been pulling their leg.
Even when Thomson used a different metal, the masses of particles emitted by the metal didn’t change. It seemed like every atom had these particles as constituents. The first thought was that a hydrogen atom, given that it is 2000 times heavier than this new electron, might be made up of 2000 or so of these electrons. But a helium atom was roughly four times the mass of a hydrogen atom. Why would the number of electrons jump from 2000 to 4000 with nothing in between? This whole-number ratio between masses of atoms in the periodic table had been one of the reasons for supposing they were truly atomic. So what could account for these discrete steps in mass? Furthermore, atoms were electrically neutral. So were there other particles that cancelled out the charge on the electron? Could you get atoms to emit positive particles to counter these negative electrons?
There was actually evidence in the experiments for a positive ray of particles running in the opposite direction. When a magnetic field was applied, they were much harder to deflect, implying that they were more massive than the electrons. The curious feature this time was that the masses of these particles seemed to vary according to the gas that was being used to fill the tubes. For hydrogen the mass was essentially the mass of the atom you started with. It seemed that the hydrogen atoms in the tube were having their electrons stripped off, leaving a large positive particle that was then attracted to the opposite electrode.
Thomson managed to achieve a similar effect with other gases: helium, nitrogen, oxygen. The masses were all whole-number multiples of the positive particle produced by the hydrogen atom. Atomic harmony yet again. As yet, there was no reason to believe that there weren’t just many sorts of positive particles, just as there were many sorts of atoms. Thomson had suggested a model of the atom known as the plum pudding. The positively charged part of the atom, which was more massive than the negative electron, formed the pudding making up the bulk of the atom, while the electrons were the tiny fruit inside.
Then the age of the bombardment of the atom began which would eventually lead to the ultimate atom smasher: the Large Hadron Collider at CERN. The New Zealand-born British physicist Ernest Rutherford is generally credited with the discovery of the proton, the particle that was the building block for all these positive particles that Thomson had investigated.
Rutherford became fascinated by the new subject of radioactivity. Uranium atoms seemed to be spitting out particles that could be picked up by photographic plates. There appeared to be two types of radiation, and these became known as alpha particles and beta particles. The alpha particles were more easily detected. Rutherford found that using a magnetic field he could deflect these alpha rays in the same way that Thomson had deflected the negative particles. Calculations showed that they had the same mass as the stripped helium atoms. Their hunch that the alpha rays being emitted by the uranium were actually bits of helium atoms was confirmed when the alpha rays were combined with a shower of electrons, which resulted in a stable gas being formed. Chemical analysis soon confirmed that the gas was indeed helium.
Читать дальше













