Mendeleev’s genius was to realize that if sometimes the elements didn’t quite match up, it perhaps indicated a missing element. The gaps in his table were probably his most insightful contribution. The fact that there was a hole in the 31st place of his table, for example, led Mendeleev to predict in 1871 the existence and properties of a new substance that would later be called gallium. Four years later French chemist Lecoq de Boisbaudran isolated the first samples of this new atom, predicted thanks to the mathematical patterns discovered by Mendeleev.
Here then was a list of the atoms that were meant to make up all of matter. For example, my dice is made from putting together carbon atoms, oxygen atoms and hydrogen atoms into a structure called cellulose acetate. My own body is predominantly made up of combinations of these atoms but with a different structure. The cellulose acetate is a homogeneous structure free from bubbles, which makes it more likely to be fair. The more antique dice were made from a nitrate-based cellulose concocted by John Wesley Hyatt in 1868. His cocktail of nitric acid, sulphuric acid, cotton fibres and camphor produced an impressive substance with great tensile strength that resisted the effects of water, oils and even diluted acids.
Hyatt’s brother named it celluloid and it became a highly cost-effective substitute for objects that had previously been carved out of ivory or horn. Billiard balls and removable collars, piano keys and even my dice were made from this synthetic plastic. The dice that were made from cellulose nitrate were the industry standard in the early twentieth century, but after several decades of use they would almost instantaneously crystallize and decompose, crumbling in on themselves and releasing nitric acid gas.
The real collectors’ dice are those Vegas dice made from cellulose nitrate in the late Forties that avoided this crystallization. My dice won’t suffer the same fate. Here is a picture of how the atoms are put together inside my dice.
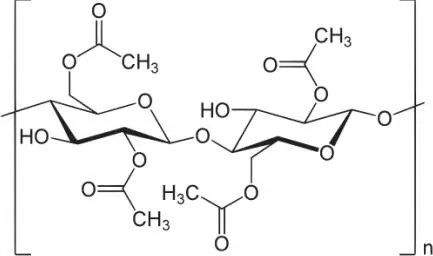
The identification of these elements was not a proof of a discrete model of matter. There was no reason that this picture of the ingredients of my dice couldn’t be a formula for the way a continuous structure combines. Although the chemists were tending towards an atomistic view of the universe, this was far from the case in the physics community. Those, like the German physicist Ludwig Boltzmann, who proposed atomic models of matter were laughed out of the lab.
Boltzmann believed that this atomic theory was a powerful way to interpret the concept of heat based on the idea that a gas was made up of tiny molecules bashing around like a huge game of micro-billiards. Heat was just the combined kinetic energy of these tiny moving balls. Using this model, combined with ideas of probability and statistics, he was successfully able to explain the large-scale behaviour of a gas. But most physicists were still committed to a continuous view of matter and were very dismissive of Boltzmann’s ideas.
Boltzmann was so ridiculed that he was forced to retreat from his belief that this billiard-ball theory of matter represented a true picture of reality, and instead was obliged to refer to it as a heuristic model if he wanted to get his ideas in print. As Ernst Mach, his great nemesis in the debate about the reality of atoms, declared mockingly: ‘Have you ever seen an atom?’
Boltzmann was plagued by fits of depression, and there is evidence that he was in fact bipolar. The rejection of his ideas by the scientific community is believed to have contributed to the depression that struck in 1906 and that led to him hanging himself during a holiday with his family near Trieste while his daughter and wife were out swimming.
It was a tragic end, not least because the most convincing evidence that he was right was just emerging. And it was one of the big names of physics who produced ideas that supported the atomistic view and were very hard to ignore. The work that Einstein and others did on Brownian motion would prove extremely difficult to explain for those who, like Mach, believed in a continuous view of the world.
Although conventional microscopes don’t allow one to see individual atoms, they did allow scientists in the nineteenth century to see the effect that these atoms were having on their surroundings. It’s called Brownian motion after Robert Brown, who in 1827 noticed the random behaviour of small particles of pollen floating on the surface of water. Since pollen was organic, Brown’s first thought was that it might be exhibiting signs of life as it jumped around the surface. A similar random behaviour of coal dust floating on alcohol had also been observed by Dutch scientist Jan Ingenhousz in 1785. When Brown saw the pollen’s behaviour replicated by inorganic matter, he was rather stumped as to what was causing the jittery motion.
It’s striking that the idea that it might be invisible atoms bashing into the larger visible material had been suggested by the Roman poet Lucretius in his didactic poem On the Nature of Things :
Observe what happens when sunbeams are admitted into a building and shed light on its shadowy places. You will see a multitude of tiny particles mingling in a multitude of ways … their dancing is an actual indication of underlying movements of matter that are hidden from our sight … It originates with the atoms which move of themselves. Then those small compound bodies that are least removed from the impetus of the atoms are set in motion by the impact of their invisible blows and in turn cannon against slightly larger bodies. So the movement mounts up from the atoms and gradually emerges to the level of our senses, so that those bodies are in motion that we see in sunbeams, moved by blows that remain invisible.
This was written in 60 BC, but it would take Einstein’s mathematical analysis of the motion to confirm this atomic explanation of the random movement in Lucretius’ sunbeams and Brown’s pollen.
The goal is to provide some model that will produce the strange motion exhibited by the small pieces of pollen on the surface of the water. If you divide the surface into a grid, there seems to be an equal probability that the pollen will move left–right–up–down. It is similar to the motion of a drunken man who randomly makes steps according to the toss of a four-sided dice. The picture below shows the paths of various particles of pollen as plotted by the French physicist Jean Baptiste Perrin, who took up the challenge of explaining the pollen’s motion in his book Les Atomes .
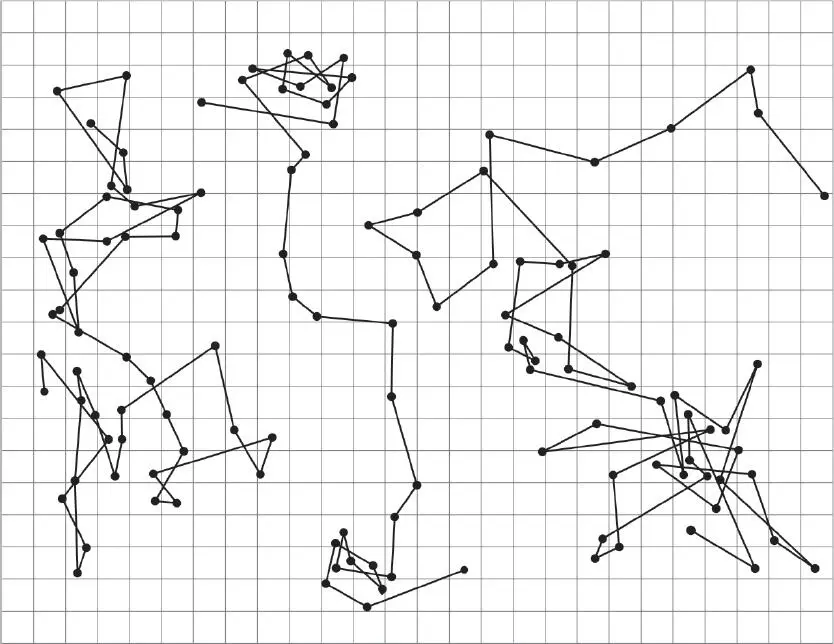
The proposal emerged at the beginning of the twentieth century that scientists were observing the pollen being buffeted by the motion of much smaller molecules of water.
It was Einstein’s mathematical brilliance that allowed him to analyse this model in which a large object was subjected to the impact of much smaller objects that were moving randomly. He proved that the model predicted precisely the observed behaviour. Think of an ice rink with a large puck sitting in the middle of the rink and then introduce a whole system of tiny pucks that are set off in random directions at particular speeds. Every now and again the tiny pucks will hit the large puck, causing it to move in one direction. The skill was to assess how many small pucks you would need, and their relative size, in order to produce the observed behaviour of the larger puck.
Читать дальше














