Various binders are available. It is important that the binder does not contain any analyte elements. Binders usually are mixed into the sample during the last grinding process; they are ground with the sample material and thus homogenized. These additives often also support the grinding process by preventing particle aggregation. The binders are available as pellets with a defined weight; therefore, no additional weighing is necessary during portioning binder and sample. This is particularly important for an automation of workflows. Liquid binders are also used; they are often the only way to produce a stable pellet. In this case, it is necessary to check whether these binders dissolve elements from the sample; then the evaporation of the solvent can lead to a depletion of these elements.
The selection of an optimum binder as well as the mixing ratio with the sample for a given analytical problem usually requires tests, since there is a dependence between sample material, grain size distribution, and the available preparation technique. Table 3.8shows a summary of the most commonly used binders.
Table 3.8 Binders and additives for pressed pellets.
| Binder |
Function |
Properties/application |
| Boron acid Borax |
Additive and binder Also advantageous for sample stabilization as sample mold |
No longer allowed, slightly toxic |
| Paraffin wax |
Mostly binder |
Slightly toxic, no influence by moisture |
| Cellulose |
Mostly binder |
Absorption of moisture |
| Methylmetaacrylate in a solution of acetone |
Binder for materials that enlarge their volume due to water absorption |
Mixing with the sample and wait for evaporation of the acetone, then pressing |
| Polyvinyl alcohol solution |
Additive and binder, avoids aggregation of the grounded material and cools down the mill |
|
The quality of sample preparation influences the analytical accuracy and the reproducibility of the analyses. The manufacturing of pressed pellets produces a much higher quality measurement sample compared to the simple loose powder samples of small size materials; it improves the analytical accuracy. For mineralogical material it is in the order of 0.5% for main components and <1% for secondary components.
3.4.4 Preparation of the Sample by Fusion Beads
3.4.4.1 Improving the Quality of the Analysis
The manufacturing of pressed pellets is fast and relatively easy; however, there still can be effects that influence the analytical accuracy, for example,
grain size effects
mineralogical effects
preferential orientations
surface roughness and
segregations of the material.
The elimination of these effects is possible by the manufacturing of fusion beads. This type of sample preparation also results in a standardization of the matrix, since a considerable dilution of the sample material by the melting agent takes place. As a result, calibrations can be used for a wider range of sample qualities, which also means less reference samples are required for a standard-based calibration. Further, calibration samples can be produced synthetically from pure substances in the form of fusion beads, so that their traceability is possible. The influence that the preparation of the material has on the analytical accuracy is demonstrated in Figure 3.12, which shows the calibration curve for pressed pellets and fusion beads.
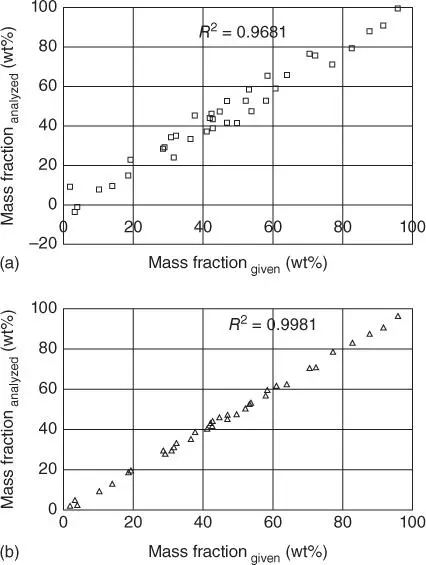
Figure 3.12 Calibration curves for the same powder material prepared as pressed pellet (a) and fusion bead (b).
The following materials are required for the manufacturing of fusion beads:
Crucible (platinum/gold 95/5)
Chill molds (platinum/gold 95/5), nickel discs
Cover for the molds (platinum/gold 95/5)
Stirring bar (e.g. platinum/gold 95/5)
Muffle furnace
Fully or partially automated fusion machine heated inductively or with burners
The disadvantages of manufacturing fusion beads are the relatively long processing times, the higher costs, and the dilution of the sample materials, since this often leads to a reduction in the detection limits.
3.4.4.2 Steps for the Production of Fusion Beads
The production of a fusion bead takes place in several steps. There are different detailed descriptions of the advantages and procedures for the manufacturing of fusion beads (Willis 2010; Claisse 1957):
First, the sample material should be ground. Here the grain sizes are not required to be as small as for pressed pellets. Grain sizes <63 μm are usually sufficient.
As pre-treatment the material is often dried by annealing. This takes place usually at temperatures of approximately 950 °C, in the case of refractory materials in accordance with ISO 12677 at 1025 °C, and should be carried out until the material mass is constant. For this purpose, the samples are placed in an Al2O3 dish and heated in a muffle furnace. In this case, the loss of ignition (LOI) must be taken into account from the evaporation of moisture or volatile materials; see Section 3.4.4.3.
Pre-oxidation of the sample material may be necessary if the sample contains pure metals that can attack or alloy with the crucible material, which can lead to a reduction in its melting point and thus damage the crucible material. The following oxidizing agents are used:NH4NO3 for low concentrations of reducing materials (oxidation at temperatures of approximately 300 °C for 1–2 minutes)LiNO3 for slags from steel production (oxidation at temperatures of approximately 815 °C for 5 minutes)NaNO3 for metal sulfides (oxidation at temperatures of 700 °C for 15 minutes). In this case, the nonvolatile Na has to be taken into account in the subsequent analyses orSrNO3, which not only oxidizes the material but also increases the mass absorption of the fusion bead.Further protection of the crucible material is possible by a layering of the oxidizing agent and the sample material, so that the crucible material only comes into contact with the melting agent, as shown in Figure 3.13.
As melting agent, usually borates are used because they cannot be detected analytically by XRF and they reduce the melting point of the samples. Most commonly, lithium metaborate and lithium tetraborate are used depending on the sample composition. Samples with high contents of Al, Si, S, or Fe react better with metaborate, and samples with high contents of Na, Mg, K, and Ca better with tetraborate. Mixtures of these two melting agents are also frequently used. Further melting agents such as sodium tetraborate are available for special applications, in order to further reduce the melting point. It must be ensured that analyte elements are not also contained in these materials. The usual fluxes as well as their melting temperature can be found in Table 3.9.
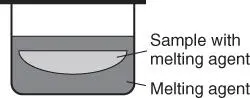
Figure 3.13 Arrangement of layers of melting agent and sample in the fusion mold.
Table 3.9 Flux agents for different applications.
| Flux |
Chemical notation |
Melting temperature (°C) |
| Lithium metaborate |
LiBO 2 |
850 |
| Lithium tetraborate |
Li 2B 4O 7 |
925 |
| Mixtures |
LiBO 2+ Li 2B 4O 7 |
825 for a 50 : 50 mix |
| Sodium tetraborate |
Na 2B 4O 7 |
741 |
Table 3.10 Sample–flux ratios for typical materials in grams (valid for diameter of the fused bead of 32 mm).
Читать дальше