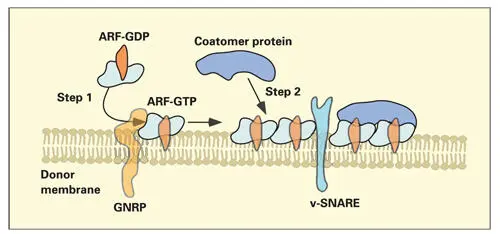
Fig 2-13Formation of a coatomer-coated membrane. The first step involves the insertion of guanine nucleotide–releasing protein (GNRP) into the membrane of the donor compartment. In the second step, GNRP reacts with adenosine diphosphate ribosylation factor (ARF), converting ARF–guanosine diphosphate (GDP) to ARF–guanosine triphosphate (GTP). Attached to the donor membrane, ARF-GTP is then able to bind coatomer proteins. As more coatomer proteins are bound to the site, a vesicle will start to form from the donor compartment by a budding process. Soluble N -ethylmaleimide–sensitive fusion attachment protein receptors (SNAREs) project from the surface of the transport vesicle (v-SNARE).
In step two, segments of the membrane covered by ARF-GTP favor the recruitment and attachment of coatomer proteins (see Fig 2-13). In mechanisms yet to be clarified, the coatomer-coated membrane is deformed and pinched off to form a coatomer-coated transport vesicle ( Fig 2-14). Transport vesicles retain their coatomer coats until they begin docking to the appropriate target membrane.
Sorting products to their appropriate destinations requires specific signals to control the docking of transport vesicles with the correct target compartment. This is accomplished by transmembrane proteins that act as surface markers. The transmembrane interacting proteins are soluble N -ethylmaleimide–sensitive fusion attachment protein receptors (SNAREs). Terrian and White reviewed the evolution of SNARE proteins and their role in traffic regulation (see Fig 2-14). 197Specific surface markers (t-SNARES) have been identified in the membranes of the RER, Golgi complex, endosomes, and plasma membrane.
Docking of the transport vesicle to the target membrane occurs when vesicle SNAREs (v-SNAREs) bind to their “target” membranes (t-SNAREs) (see Fig 2-14). Rab-GTP, a second type of monomeric guanosine triphosphatase, present in the vesicle membrane, functions as a monitor and stabilizer of the fit between the two types of SNARE molecules. A guanosine triphosphatase–activating protein in the target membrane causes ARF-GTP to hydrolyze GTP to GDP. In its GDP-binding state, ARF retracts its fatty acid anchor and detaches from the vesicle membrane. Simultaneously, the ARF-coatomer complex disassembles.
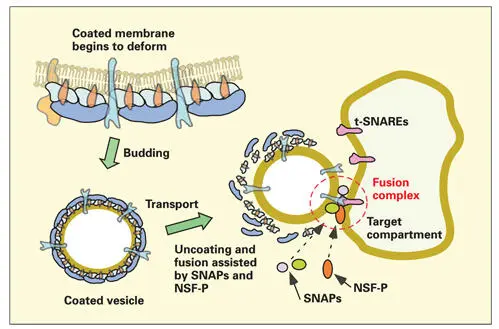
Fig 2-14Formation, docking, and fusion of the transport vesicle. The coatomer-coated vesicle, with soluble N -ethylmaleimide–sensitive fusion attachment protein receptor (v-SNARE) molecules exposed beyond the coatomer coating, is available for binding (step 2, docking reaction) to the appropriate target membrane SNARE (t-SNARE) molecules. During the docking reaction, adenosine diphosphate ribosylation factor–guanosine triphosphate is hydrolyzed to adenosine diphosphate ribosylation factor–guanosine diphosphate and disassociates from the vesicle membrane. Coatomer proteins are also released. The attachment of v-SNARE to t-SNARE is monitored and stabilized by a second type of guanosine triphosphate, a Rab-GTP molecule present in the donor vesicle membrane. Fusion of the transport vesicle membrane to the target (step 3) is induced by a protein complex that includes N -ethylmaleimide–sensitive fusion protein (NSF-P) and soluble NSF attachment proteins (SNAPs).
For membrane fusion to occur, special fusion proteins are required to displace water molecules and to overcome the electrostatic repulsive forces between the two closely juxtaposed lipid membranes. 198The space between the adjacent membranes must be reduced to less than 1.5 nm. The v-SNARE to t-SNARE receptor-ligand docking reaction between the transport vesicle and its target membrane recruits fusion proteins to the site of attachment. N -ethylmaleimide– sensitive fusion (NSF) protein and soluble NSF attachment proteins (SNAPs) have been shown to carry out fusions in eukaryotic cells by interacting with SNAREs (see Fig 2-14). Conformational changes in the fusion protein, driven by ATP, destabilize the lipid membranes, leading to the formation of a fusion pore that rapidly expands to permit total fusion of the two membranes. Exactly how this machinery is assembled and how it functions during the fusion event is still speculative.
Application of this new knowledge of the regulation of cytoplasmic traffic must be applied to future studies of the odontoblastic Golgi complex to gain a clearer understanding of the secretion and retrieval of dentin matrix components.
From the trans-Golgi network, there are two basic pathways of secretion: the regulated pathway and the constitutive pathway. 199 , 200In the regulated pathway, secretory product is stored in vesicles or granules until secretion is triggered by an appropriate signal. Secretory granule formation involves condensation of the secretory product from larger condensing vacuoles (presecretory granules) considered to be part of the trans-Golgi network (see Fig 2-13). 201Budding of membrane from the condensing vacuole continues, until a smaller and denser secretory granule is formed. In the constitutive pathway, products are exported immediately after they are packaged in the Golgi apparatus.
Regulated secretion requires an intact microtubular system to transport granules to a specific region of the cell surface, usually the apical end of the cell. Constitutive secretion does not appear to be dependent on microtubules and may occur from many regions of the cell surface.
Microtubules form a radiating network, extending from the centrosome outward to the cell periphery. This network provides a structural pathway for the translocation of secretory granules. The energy source for granule transport is derived from the hydrolysis of ATP. Enzymatic motor proteins (mechanochemical ATPases) associated with the granule membrane hydrolyse ATP molecules when activated by contact with the microtubules.
Cyclical attachment and detachment produces movement of the granule along the length of the microtubule. The mechanochemical ATPase responsible for anterograde movement of secretory granules is a member of the kinesin family of motor proteins. Substances that interfere with microtubule assembly, such as antimitotic (antispindle) agents, and substances that deplete ATP or stop its production produce abnormalities in deposition of dentin, enamel, and bone matrices.
Microtubule-directed transport delivers secretory granules to the periphery of the secretory pole of the cell. At that point, further transport toward the plasma membrane is dependent on myosin. The final approach and fusion is both nonmicrotubule and nonmyosin dependent. 202Discussion of microtubules is continued in chapter 3.
A proposed pathway for secretion in the odontoblast, based on ultrastructural studies of the odontoblast and the current theories of Golgi organization, is outlined in Fig 2-15. Additional studies of odontoblast structure and function are needed to determine whether there are multiple forms of secretory granules in odontoblasts and to identify the signals that direct secretory granule discharge.
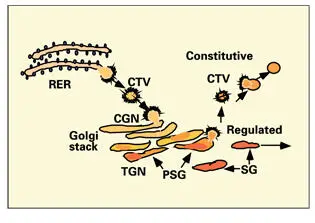
Fig 2-15Odontoblast secretory pathway. Intermediate coated transport vesicles (CTV) bud from the rough endoplasmic reticulum (RER) and migrate to the cis-Golgi network (CGN), where they fuse with the outermost cisternae of the Golgi stack. Presecretory granules (PSG) form part of the trans-Golgi network (TGN). Concentration of the secretory product occurs by aggregation of proteins inside the PSGs and by the removal of fluid and membrane via budding of small vesicles. Vesicles containing membrane proteins and lipids are secreted in the constitutive pathway. Matrix secretory granules (SG) are transported via the regulated pathway, along microtubules, to the odontoblastic process.
Читать дальше