Clinical Correlations
Secondary, tertiary (reactive), and reparative dentin
Odontoblasts are nondividing cells with a long life span. During tooth development, they produce primary dentin at the rate of about 4 to 8 μm per day. Once the crown is completed and the apical length of the root has been established, odontoblasts produce secondary dentin at 1 to 2 μm per day. Histologic studies of human teeth have shown that all teeth contain secondary dentin. It is deposited throughout life as long as the pulp remains vital.
Although there are no reliable data on the estimated longevity of individual odontoblasts, the continued slow deposition of secondary dentin suggests that odontoblasts are long-lived cells. Odontoblasts lose nearly half of their RER and Golgi profiles following formation of primary dentin. 203Biochemical studies indicate that, on completion of primary dentin, there is an 80% reduction in alkaline phosphatase activity at the predentin-odontoblast region, and a concomitant reduction of ATPase activity in the odontoblasts. This reduced metabolic function is consistent with a slow production of secondary dentin.
Secondary dentin is characterized by a regular arrangement of dentinal tubules, usually in direct continuity with those of the primary dentin. Micro-hardness measurements indicate that secondary dentin is about 30% to 40% softer than primary dentin. The biochemical and matrix factors responsible for the decrease in mineral content have not been identified.
Tertiary or reactive dentin is produced in response to nonlethal irritation of the odontoblasts. Once activated, either by a slowly progressing carious lesion, dental abrasion, or restorative dentistry procedure, odontoblasts resume deposition of dentin at rates that approach those measured for formation of primary dentin. Reactive dentin is deposited subjacent to the area of injury as a protective barrier for the pulp.
Reactive dentin does not have the well-organized histologic structure of primary or secondary dentin. The dentinal tubules are fewer and less likely to be neatly parallel to each other. A calciotraumatic line is commonly found to separate the secondary dentin from the reactive dentin. Excessive formation of reactive dentin in the root portion of the pulp can lead to varying degrees of pulp canal obliteration, a condition that complicates pulp canal therapy.
Recent studies of superficial carious lesions, where the zone of demineralization had not reached the dentinoenamel junction, demonstrated increased deposition of peritubular dentin and a decreased width of the predentin. 204With progression of the lesion to the dentinoenamel junction, the predentin grew wider than control predentin as deposition of collagen increased. Cell proliferation in the subodontoblastic layer accompanied changes in activity of the odontoblasts.
It was suggested that in early enamel caries, the odontoblasts respond to stimuli transmitted along partially demineralized enamel rods and the dentinal tubules. 204When the caries process involves dentin, fibronectin and a 165-kDa fibronectin-binding protein are deposited on the surface of the odontoblastic process and along the walls of the dentinal tubules. 205It was suggested that fibronectin and 165-kDa proteins regulate reactive dentinogenesis, perhaps by playing a signaling role similar to that which occurs during initial odontoblast differentiation.
If the injury is severe, as in rapidly advancing dental caries or in a dental operative procedure producing excessive heat, the odontoblasts undergo necrosis. In this case, repair (the formation of reparative dentin) must await the differentiation of new odontoblast-like cells from precursors, either from the cell-rich zone or from deeper regions of the pulp. 206The presence of a fibronectin-rich surface, permitting adhesion of pulp cells, appears essential to the differentiation of odontoblast-like cells. 207
Replacement odontoblasts are generally produced in fewer numbers than the original complement. The reparative dentin that they deposit is characterized by irregular tubules. Under less favorable conditions, new odontoblasts may fail to differentiate, and repair is carried out by fibroblastic cells that deposit a fibrodentin type of matrix. In either case, the process requires several weeks.
Recent experiments have shown that certain growth factors, namely bone morphogenetic protein (BMP-2, BMP-4, and BMP-7), TGF-β1, and components of dentin matrix, stimulate the development of the odontoblast phenotype and the expression of type I collagen and osteocalcin in pulp cells. 208– 210 Cultured human pulp fibroblasts express BMPs and BMP receptor. 209 , 211 , 212In the presence of fibronectin, TGF-β1 and BMP-2 trigger odontoblast development from embryonic dental papilla cells. 89 , 213It has been proposed that these growth factors have a regulatory role in the initiation of reparative dentin by activating the differentiation of new odontoblasts. 214 – 216
The potential value of BMPs as pulp-capping agents is under investigation by several research groups. Recombinant human BMP-2, BMP-4, and BMP-7 have been incorporated in pulp-capping preparations applied to the pulps of dogs and monkeys. Significant increases in reparative dentin were noted after several months in teeth capped with BMP preparations. 210 , 217 – 219High–molecular weight hyaluronic acid also promotes formation of reparative dentin in amputated dental pulp. 220
Sclerotic dentin
Empty dentinal tubules result from either the physiologic retraction of the odontoblastic process or from the death of the odontoblasts. These tubules appear as dark bands (dead tracts) in ground mineralized sections when viewed under transmitted light. Open dentinal tubules, especially at the cervical region, often lead to dental hypersensitivity. Occlusion of the tubules by precipitation of calcium salts or with composite resin reduces the flow of fluid and decreases the sensation of pain. 221 – 223
During mild irritation, dentinal tubules may become obliterated by mineral deposition, a process known as dentinal sclerosis ( Fig 2-16). Some investigators have suggested that continued or excessive deposition of peritubular dentin is the basis of dentinal sclerosis. Sclerotic dentin is usually present under chronic carious lesions, dental restorations, and areas of attrition. It has been suggested that sclerosis of dentinal tubules is a defense mechanism for protection of the pulp. In ground sections, sclerotic dentin appears translucent, blending in closely with adjacent mineralized intertubular dentin. The distal portion of the odontoblastic process may become mineralized in carious dentin. 103
In the caries process, dentin demineralization begins when the enamel lesion reaches the dentinoenamel junction. The caries process does not spread preferentially along the dentinoenamel junction but rather progresses into the dentin along the dentinal tubules. 224In sclerotic tubules, bacteria advance more slowly because they must remove hydroxyapatite crystals by acid dissolution. Nevertheless, because of the small crystallites (large surface area for exchange) in sclerotic and peritubular dentin, and the absence of a collagen fibril matrix, bacteria are able to advance preferentially along the tubules (see Fig 2-16). Destruction of the collagenous matrix of intertubular dentin proceeds more slowly, because proteolytic enzymes must gain access to and degrade the collagen matrix after it has been demineralized. Bacterial invasion of the dentinal tubules is a complex process involving bacterial adhesions to extracellular matrix molecules, proteolytic enzymes, and the ability of bacteria to survive in an environment of limited oxygen and nutrients. 225
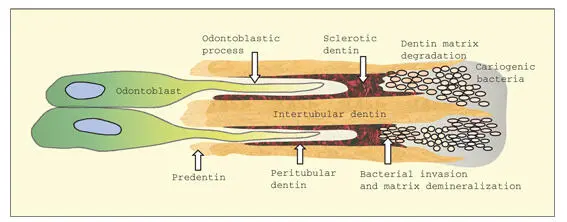
Fig 2-16In a caries lesion, cariogenic bacteria invade the dentinal tubules, demineralizing sclerotic and peritubular dentin in the process. Intertubular dentin is slower to degrade because of its dense collagenous matrix and larger hydroxyapatite crystals.
Читать дальше