1 ...8 9 10 12 13 14 ...43 Nidogen Nidogen (also called entactin ) is a rod-shaped protein consisting of a single polypeptide chain, approximately 30 nm long, with globular domains at each end and one centrally located domain. 106,107Because nidogen binds with high affinity to collagen IV and laminin, it has an organizing role in assembly of the basal lamina. Nidogen also binds perlecan, the large heparan sulfate proteoglycan of the basal lamina.
The coexpression of laminin 1 and nidogen results in a relatively stable basal lamina. In general, laminin is produced by epithelial cells and nidogen by mesenchymal cells. Temporospatial differences in the expression of laminin and nidogen are thought to have significance in epithelial-mesenchymal tissue remodeling because of resulting changes in the stability of the basement membranes. 108
Basal lamina The basal lamina is a supramolecular aggregate of type IV collagen, laminin, fibronectin, nidogen, and perlecan. They form a macromolecular network with the dual function of supporting epithelial cells and providing for a permeability barrier or filter. Meyer et al 109have reviewed the role of the basal lamina in tooth development and odontoblast differentiation. The basal lamina is discussed in detail in chapter 4.
Clinical Correlation: The Human Dentition
The primary (deciduous) dentition consists of 20 teeth, five in each quadrant ( Fig 1-22). 74 , 110The permanent incisors, canines, and premolars form from successional laminae that extend lingually from the primary precursors toward the midline (see Fig 1-22). The permanent molars develop from a distal extension of the dental lamina, the accessional lamina ( Fig 1-23). Some dental embryologists consider the permanent molars to be members of the first dentition. Their microscopic successors undergo an aborted development.
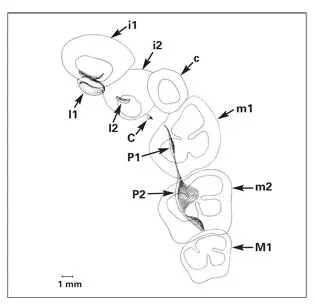
Fig 1-22Developing primary teeth and the primordia of the permanent teeth in a 28-week human fetus. Maxillary quadrant. (i1) Primary central incisor; (i2) primary lateral incisor; (c) primary canine; (m1) primary first molar; (m2) primary second molar; (I1) permanent central incisor; (I2) permanent lateral incisor; (C) permanent canine; (P1) permanent first premolar; (P2) permanent second premolar; (M1) permanent first molar. (Adapted from Ooe 74with permission.)
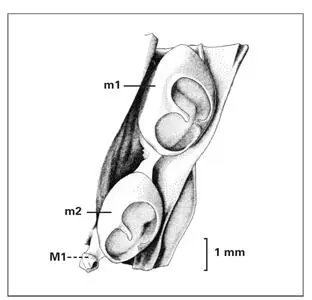
Fig 1-23Mandibular molar region in a 159-mm fetus (at 20 weeks old), depicting the formation of the permanent first molar (M1) from a distal extension of the primordia of the primary second molar (m2). (m1) Primary first molar. (Adapted from Ooe 74with permission.)
During the development of primary teeth, the central incisor and canine are positioned labial to the lateral incisor ( Fig 1-24). This arrangement is noted very early in the formation of the enamel organ from the dental lamina. The buds of the permanent teeth have a similar position, so that the lateral incisor is positioned lingual to the central incisor and canine. During normal postnatal development, space is created in the dental arch for the alignment of all anterior teeth. Often, the space created is insufficient, and the central incisor and the canine crowd out the lateral incisor.
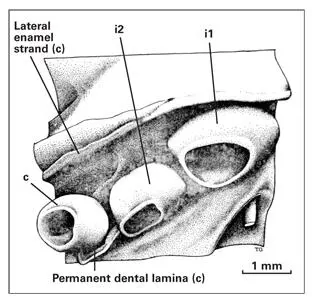
Fig 1-24Epithelial portion of the anterior tooth germs and adjacent structures in a 144-mm fetus. (i1) Primary central incisor; (i2) primary lateral incisor; (c) primary canine. (Adapted from Ooe 74with permission.)
References
1. Graveson AC, Smith MM, Hall BK. Neural crest potential for tooth development in a urodele amphibian: Developmental and evolutionary significance. Dev Biol 1997;188:34–42.
2. Lumsden AGS. The neural crest contribution to tooth development in the mammalian embryo. In: Maderson PFA (ed). Developmental and Evolutionary Aspects of the Neural Crest. New York: Wiley, 1987:261–300.
3. Thesleff I, Partaanen AM, Vainio S. Epithelial-mesenchymal interactions in tooth morphogenesis: The roles of extracellular matrix, growth factors, and cell surface receptors. J Craniofac Genet Dev Biol 1991;11:229–237.
4. Ruch JV, Lesot H, Karcher-Djuricic V, Meyer JM, Mark M. Epithelial-mesenchymal interactions in tooth germs: Mechanisms of differentiation. J Biol Buccale 1983;11:173–193.
5. Thesleff I, Vaahtokari A, Vainio S, Jowett A. Molecular mechanisms of cell and tissue interactions during early tooth development. Anat Rec 1996;245:151–161.
6. Slavkin HC, Diekwisch T. Evolution in tooth developmental biology: Of morphology and molecules. Anat Rec 1996;245: 131–150.
7. Slavkin HC. Molecular determinants during dental morphogenesis and cytodifferentiation: A review. J Craniofac Genet Dev Biol 1991;11:338–349.
8. Bronner-Fraser M. Origins and developmental potential of the neural crest. Exp Cell Res 1995;218:405–417.
9. Imai H, Osumi-Yamashita N, Ninomiya Y, Eto K. Contribution of early-emigrating midbrain crest cells to the dental mesenchyme of mandibular molar teeth in rat embryos. Dev Biol 1996;176:151–165.
10. LeDouarin NM, Dupin E, Ziller C. Genetic and epigenetic control in neural crest development. Curr Opin Gen Dev 1994;4:685–695.
11. Thomas BL, Tucker AS, Ferguson C, Qiu M, Rubenstein JLR, Sharpe PT. Molecular control of odontogenic patterning: Positional dependent initiation and morphogenesis. Eur J Oral Sci 1998;106:44–47.
12. Thomas BL, Tucker AS, Qiu M, Ferguson C, Hardcastle Z, Rubenstein JLR, Sharpe PT. Role of Dlx-1 and Dlx-2 genes in patterning of the murine dentition. Development 1997;124: 4811–4818.
13. Mina M, Kollar E. The induction of odontogenesis in non-dental mesenchyme combined with early murine mandibular arch epithelium. Arch Oral Biol 1987;32:123–127.
14. Kollar E, Mina M. Role of the early epithelium in the patterning of the teeth and Meckle’s cartilage. J Craniofac Genet Dev Biol 1991;11:223–228.
15. Lumsden AGS. Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development 1988;103(suppl):155–169.
16. Wang Y-S, Upholt WB, Sharpe PT, Kollar E, Mina M. Odontogenic epithelium induces similar molecular responses in chick and mouse mandibular mesenchyme. Dev Dyn 1998; 213:386–397.
17. Weiss K, Stock D, Zhao Z, Buchanan A, Ruddle F, Shashikant C. Perspectives on genetic aspects of dental patterning. Eur J Oral Sci 1998;106:55–63.
18. Qiu M, Bulfone A, Ghattas I, Meneses JJ, Christensen L, Sharpe PT, Presley R, Pedersen RA, Rubenstein JLR. Role of the Dlx homeobox genes in proximodistal patterning of the branchial arches: Mutations of Dlx-1, Dlx-2, and Dlx-1 and -2 alter morphogenesis of proximal skeletal and soft tissue structures derived from the first and second arches. Dev Biol 1997;185:165–184.
19. Sharpe PT. Homeobox genes and orofacial development. Connect Tissue Res 1995;31:1–9.
20. Chen Y, Bei M, Woo I, Satokata I, Mass R. Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development 1996;122:3035–3044.
21. Whiting J. Craniofacial abnormalities induced by the ectopic expression of homeobox genes. Mutation Res 1997;396: 97–112.
22. Satokata I, Mass R. Msx-1-deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet 1994;6:348–356.
Читать дальше