Yeasts are unicellular fungi ( Figure 1.6b) with great industrial importance, notably S. cerevisiae , the major yeast used in alcoholic fermentation (see Chapter 2). Yeasts are heterotrophic, and most of them can grow in the presence and absence of O 2(facultative anaerobes), unlike most fungi. Yeasts are not nutritionally demanding as a relatively simple medium composition (e.g. reduced carbon sources, organic and inorganic nitrogen sources such as urea and ammonium salts, respectively, some minerals, and water) allows them to multiply. Sometimes, vitamins (e.g. biotin) are also supplemented to allow the optimal growth of the yeasts. Yeasts of industrial importance include the genera of Blakeslea , Candida , Hansenula , Kluyveromyces , Pachysolen , Phaffia , Pichia , Rhodotorula , Saccharomyces , Xanthophyllomyces , Yarrowia , and Zygosaccharomyces (Waites et al. 2001). Thousands of examples have been reported in the literature to describe the potential of these yeasts in different industrial sectors (Defavari do Nascimento and Pickering 2017; Drévillon et al. 2018; Koubaa et al. 2020; Peris et al. 2018).
The selection of a microbial species to perform a bioprocess does not only require its ability to synthesize a potentially useful compound or to carry out a particular metabolic pathway. Indeed, most of the industries seek strains that can meet other important criteria, which will allow the optimization of the biological process and maximize profitability. Such criteria include:
1 The ability of the strain to grow quickly on inexpensive organic substrates (e.g. molasses, corn liquor, whey, etc.);
2 The ability of the strain to perform in a simple and fast way the sought‐after transformations with high efficiency and a minimum of energy consumption;
3 The strain must be genetically stable (low mutation rate) to maintain its production capacity over time;
4 The strain must be specialized in the synthesis of products that are easy to extract and separate; and
5 The strain should not be pathogenic.
Wild‐type strains are usually unable to meet the above‐mentioned criteria. Indeed, they often have a limited performance that must be amplified to reach the industrial requirements. In fact, wild‐type microorganisms have usually metabolic regulation mechanisms, often of the negative feedback type, allowing them to produce naturally only the quantity of enzymes and metabolites they need to survive in a competitive environment. By short‐circuiting these mechanisms with genetic modifications, more productive microorganisms could be generated. Besides, the wild strain may have certain undesirable characteristics that can also be modified, such as sensitivity to a bacteriophage, the trend to generate a lot of foam in a liquid medium, or the synthesis of a by‐product that is difficult to eliminate during the downstream purification steps. In all cases, there is an interest in implementing a genetic improvement program for the strain involving the use of mutations or recombinant DNA technology ( Figure 1.7). Further reading about microbial transformation techniques can be found in Han (2004). The review of the literature showed hundreds of examples of genetic modifications of microbial strains toward their use in industrial bioprocesses. The major metabolic engineering achievements in the recent years include microbial production of amino acids (e.g. L‐valine, L‐threonine, L‐lysine, and L‐arginine), bulk chemicals (e.g. 1,4‐butanediol, 1,4‐diaminobutane, 1,5‐diaminopentane, 1,3‐propanediol, butanol, isobutanol, and succinic acid), and drugs (e.g. artemisinin) (Lee and Kim 2015).

Figure 1.7 Diagram of a typical gene cloning methodology and cell banking system preparation.
1.4 Fermentation Technology
The main challenge facing bioprocess specialists is the technological transfer from the laboratory to the industrial scale. Indeed, it is usually quite simple to cultivate a microorganism in an Erlenmeyer flask containing a few hundred milliliters of culture medium. However, the same operation is much more complicated in industry, where high culture volumes are processed. Nonetheless, whether in the laboratory or the industry, certain steps must be taken to cultivate a microorganism and make it produce a molecule of commercial interest.
In this regard, the fermentation industry provides a basic model in which a sequence of steps is commonly followed in the majority of bioprocesses. Whatever the strain used and the product sought, there are six major points to take into consideration when developing an industrial fermentation:
1 The formulation of an adequate culture medium that will promote the growth of the strain used and the production of the molecule of interest;
2 Sterilization of the culture medium, the bioreactor, and accessory components to prevent another microorganism developing there and contaminating the fermentation;
3 The development of an inoculum that will be used to produce a pure culture of the selected strain in sufficiently high concentration and volume to be able to adequately inoculate the bioreactor;
4 The growth of the strain within the bioreactor, under optimal and controlled production conditions;
5 Recovery and purification of the product from the culture (i.e. downstream processing steps); and
6 The elimination of effluents and other residues from the process.
The inoculum represents the biomass (e.g. cells, spores, mycelium, etc.) required for seeding the culture in a bioreactor. On one hand, it must provide a sufficient quantity of cells in homogeneous suspension to achieve rapid growth in the bioreactor, while, on the other hand, it must be free from any microbial contamination. To achieve this, an industrial lyophilized or frozen strain from a working cell bank ( Figure 1.7) is used and grown in a liquid medium stirred in an Erlenmeyer flask to obtain a significant volume of culture. This volume will be then transferred aseptically to a small bioreactor, commonly called pre‐culture , which has the function of developing a very high and metabolically active cellular biomass concentration. These stages may require several days of work and, in the case of fermentation, are carried out in very large volumes; it is often even a real fermentation before the main fermentation. Generally, the volume of the inoculum represents between 5 and 10% of the volume of the main bioreactor where the fermentation will take place. Seeding steps at an industrial scale are presented in Figure 1.8.
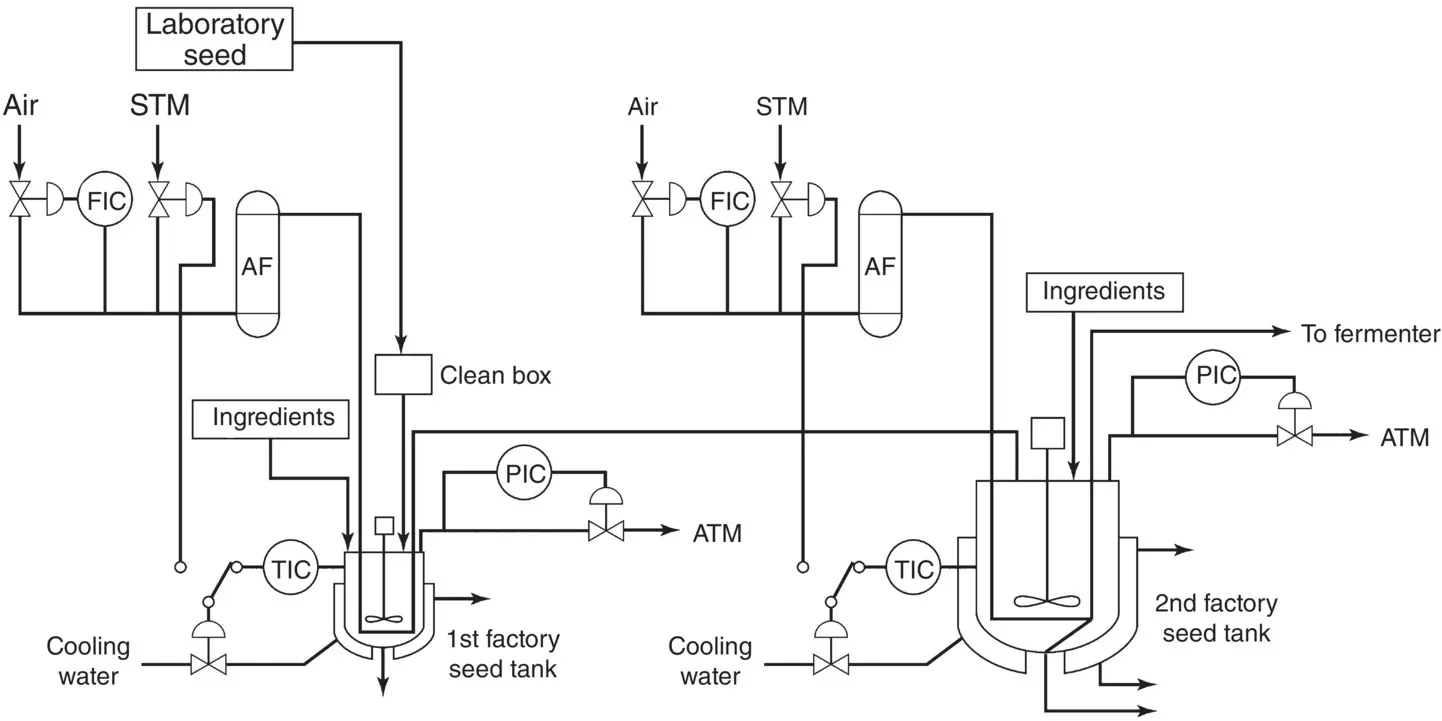
Figure 1.8 Factory seed culture equipment. AF = air filter; FIC = flow indication control; STM = steam; TIC = temperature indication control; PIC = pressure indication control; ATM = atmosphere.
Source: Oka (1999).
In the field of industrial fermentation bioprocesses, developing a profitable process requires the consideration of some criteria for the culture medium:
1 The environment must be as inexpensive as possible (i.e. the cost of acquiring and storing raw materials must be affordable).
2 Raw materials should be available on an annual basis and ideally in the local market.
3 The quality of the raw materials must be constant, which allows obtaining similar results in terms of yield and productivity between the different batches.
Читать дальше














