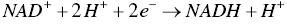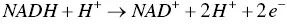Biochemical assimilation (anabolism) and dissimilation (catabolism) of nutrients by a chemoorganotroph are mediated by a network of enzymatic reactions perfectly synchronized and regulated. Anabolic pathways consist mainly of the reductive processes that lead to producing new cellular molecules, while catabolic pathways include the oxidative processes involved in removing electrons from substrates or intermediates that are used to generate energy.
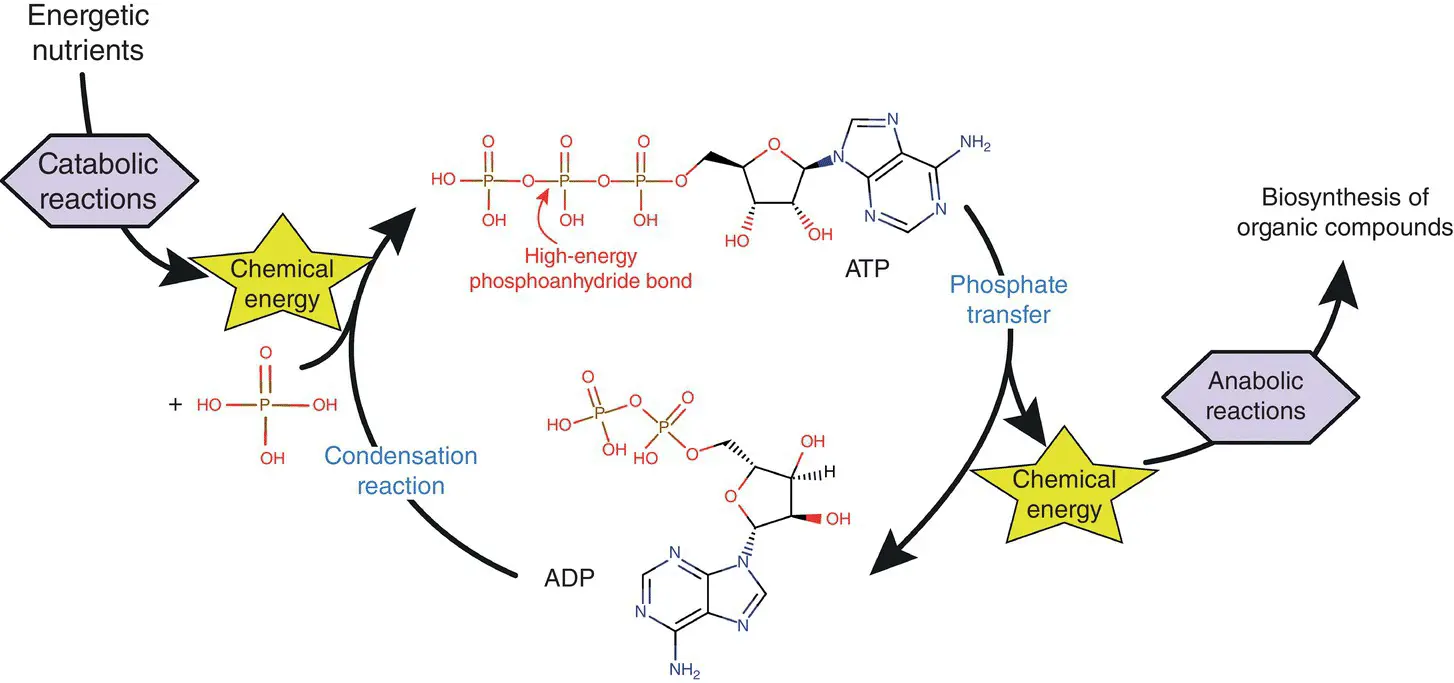
Figure 1.1 Energy coupling and the role of ATP in microbial metabolism. ADP: adenosine diphosphate, ATP: adenosine triphosphate
1.2.1 Energy Transfer and Redox Reactions
The energy released by the catabolic reactions is associated with the electrons of molecules that are degraded during these reactions. This energy is transferred to a molecule of ADP to form ATP. More specifically, a phosphate group is added to the ADP molecule, with an energy investment, to form an ATP molecule ( Figure 1.1). The energy contained in organic molecules cannot be released all at once; otherwise, it would be practically all lost in the form of heat, as during combustion. It must, therefore, be gradually transferred to the ATP molecules via the cascades of redox reactions.
First, the energetic nutrients are oxidized, which allow them to behave as electron donors (e −). During this reaction, two electrons and two protons (H +) are transferred to a coenzyme known as nicotinamide adenine dinucleotide (NAD +) to be reduced in the form of NADH + H +. A part of the chemical energy contained in nutrients is then transferred to the NAD +by the electrons, according to the reduction reaction in Eq. (1.1).
(1.1) 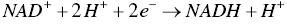
Overall, each time an organic molecule is oxidized (the loss of electrons and H +ions), there is simultaneously a reduction of NAD +taking place. This is why we talk about “redox reactions.” The newly formed NADH will then undergo oxidation, in turn, to release stored energy ( Eq. (1.2)), which will eventually be transferred to ATP molecules by various chemical processes.
(1.2) 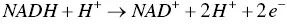
At the end of the energy transfer process, the released electrons and protons (H +) must be picked up by a final acceptor. This acceptor will vary according to the preferred catabolic pathway: aerobic respiration, anaerobic respiration, or fermentation.
1.2.2 Aerobic Respiration
In this catabolic pathway, the final electron acceptor is molecular oxygen (O 2), and the organisms using it are, therefore, dependent on air for their survival. It takes place in three stages, each involving a series of chemical reactions: glycolysis, citric acid cycle, and electron transport chain.
1.2.2.1 Glycolysis Pathway
In the glycolytic pathway, occurring in all tissues, glucose is oxidized to provide energy (i.e. ATP) and intermediates for other cellular metabolic pathways. Glycolysis is at the hub of carbohydrate metabolism where glucose is converted to pyruvate following a series of 10 enzymatic reactions ( Figure 1.2). This metabolic pathway is known as aerobic glycolysis , as the reoxidation of the NADH formed during the oxidation of glyceraldehyde 3‐phosphate requires O 2. Aerobic glycolysis sets the stage for the oxidative decarboxylation of pyruvate to acetyl coenzyme A (acetyl‐CoA), a major fuel of the tricarboxylic acid cycle (Ferrier 2017).
During glycolysis, two ATP molecules are initially consumed to phosphorylate glucose, which thus receives an essential energy supply to continue the catabolic pathway. Subsequently, two molecules of NAD +oxidize phosphorylated sugar and are reduced to NADH + H +. During these reactions, which will lead to the synthesis of two molecules of pyruvic acid from a glucose molecule, a part of the energy released allows the direct synthesis of four molecules of ATP. Considering that two molecules of ATP are consumed and four are produced, glycolysis presents a net balance of two molecules of ATP for each molecule of oxidized glucose.
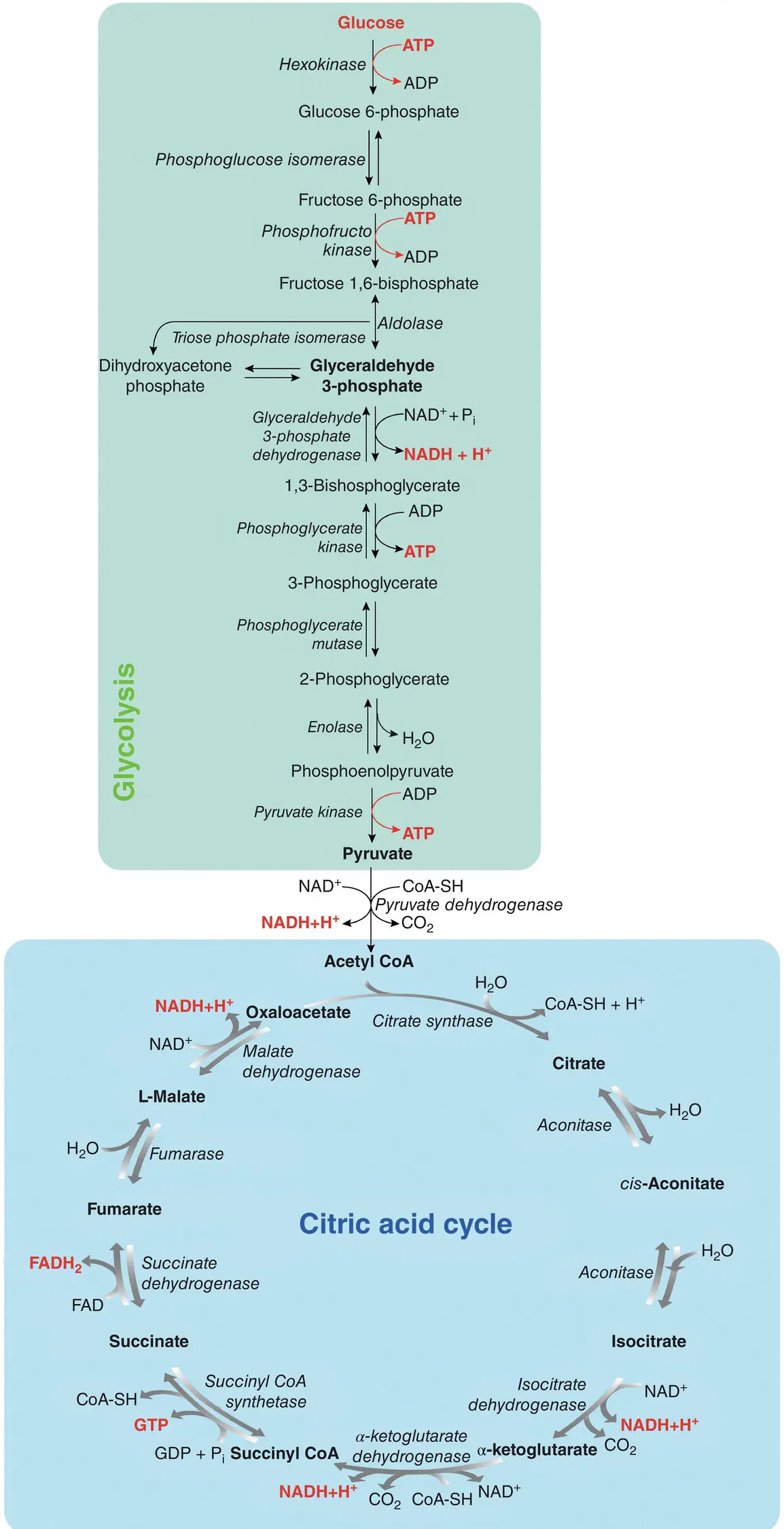
Figure 1.2 Glycolysis and citric acid cycle pathways.
1.2.2.2 Citric Acid Cycle
Pyruvic acid is first decarboxylated (release of CO 2) and then oxidized by a molecule of NAD +(reduced to NADH + H +) to form the two carbon molecules of acetyl‐CoA. As two molecules of pyruvic acid are produced from a glucose molecule, two molecules of CO 2, NADH + H +, and acetyl‐CoA are produced. In prokaryotic cells (e.g. bacteria), the citric acid cycle (or Krebs cycle) occurs in the cytosol, while in eukaryotic organisms (e.g. yeast), it takes place in the mitochondrial matrix.
Overall, two molecules of CO 2are produced and three molecules of NAD +are reduced to NADH + H +during a turn of the citric acid cycle. Besides, two electrons and two protons released from pyruvic acid are used to reduce a molecule of flavin adenine dinucleotide (FAD) into FADH 2, similar to that of NAD +. Finally, there is a release of chemical energy, which allows the synthesis of an ATP molecule. To summarize, for each molecule of glucose oxidized, four molecules of CO 2, six molecules of NADH + H +, two molecules of FADH 2, and two molecules of ATP are generated.
1.2.2.3 Electron Transport Chain and Oxidative Phosphorylation
In a process called oxidative phosphorylation , the energy stored in the reduced coenzymes (NADH and FADH 2) is released to produce ATP molecules. During this process, each coenzyme is oxidized and gives its two energy‐rich electrons to an electron transport chain, or respiratory chain, located in the plasma membrane in prokaryotes and the inner membrane of mitochondria in eukaryotes. The chain consists of complex organic acceptors that transfer electrons through a series of redox reactions ( Figure 1.3). As the electrons are transferred in this chain, the energy that they contain is released by stages and is used to activate chemiosmosis. This creates a gradient of H +protons whose energy is reinvested, through the enzyme ATP synthetase, in the production of three molecules of ATP per molecule of oxidized NADH and two molecules of ATP per molecule of oxidized FADH 2. At the end of the transport chain, the electrons exhausted of their energy bond with O 2and H +ions to form a water molecule. To summarize, for each glucose molecule that enters glycolysis, 34 molecules of ATP will be produced by oxidative phosphorylation (i.e. 30 molecules coming from NADH and 4 coming from FADH 2). Further reading about the electron transport chain could be found in Campbell (2015).
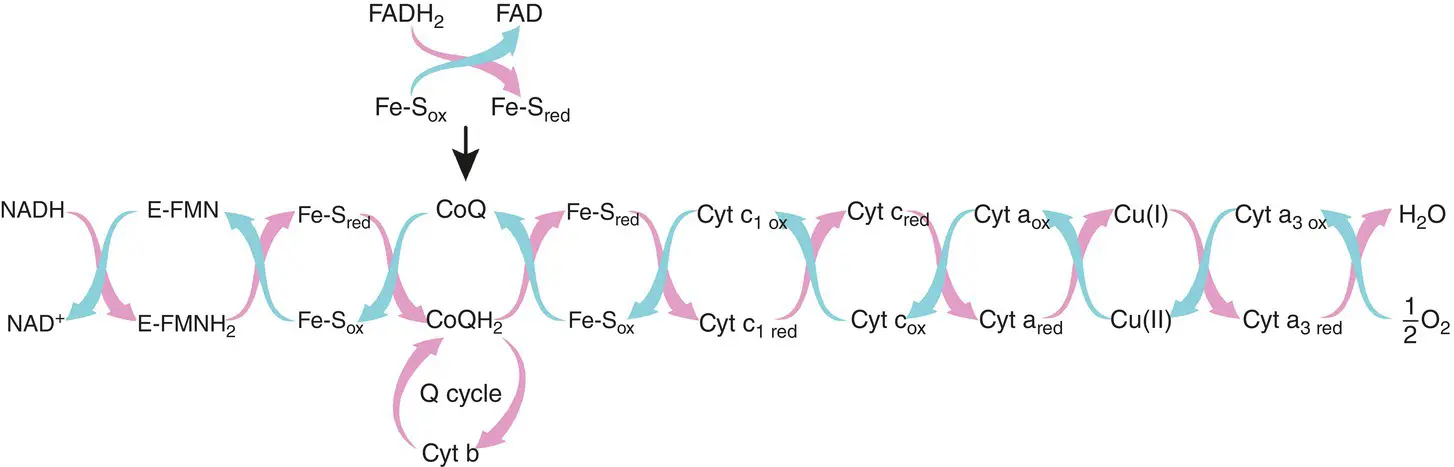
Figure 1.3 The electron transport chain showing the respiratory complexes.
1.2.3 Anaerobic Respiration
Some microbial species use anaerobic respiration to obtain their energy in the absence of O 2. During this process, the electrons that are removed from organic nutrients such as glucose follow the same pathways as in aerobic respiration, except that the final acceptor is not O 2, but another inorganic molecule (e.g. sulfate, nitrate, etc.). The sulfate ion is generally reduced to hydrogen sulfide, while nitrate ion can be reduced to nitrite, nitrogen oxide, or molecular nitrogen. Some bacteria reduce carbonate to methane. The number of ATP molecules produced by anaerobic respiration varies from one organism to another and from one metabolic pathway to another. This number is generally less than the 38 mol of ATP generated by aerobic respiration, the energy yield is lower, and anaerobic microorganisms usually grow slower than aerobic ones.
Читать дальше